MACROMOLECULES Four Classes of Organic Molecules: Lipids
advertisement

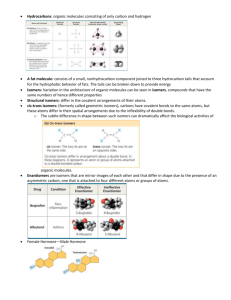
MACROMOLECULES Four Classes of Organic Molecules: Lipids, Carbohydrates, Proteins, Nucleic Acids Organic molecules contain carbon and hydrogen. Covalently bound Based on carbon chains or rings The diversity of organic molecules makes the diversity of life possible. Remember molecules have shapes Polymers (Greek = many parts), composed of (usually) many monomers Formed by condensation synthesis, or dehydration – the net loss of a water molecule Disassembled by hydrolysis (Greek = breaking with water) With the help of specific enzymes Isomer (Greek = same part): Same molecular formula but different atomic arrangement, shape Functional Groups: determine polarity and reactivity Carbohydrates: C, H, O Monosaccharides, disaccharides, polysaccharides; generally some multiple of CH2O Simple sugars (one ring) or polymers of sugars Glucose: E now, Polysaccharides: stored E Glycogen: stored E in animals, Starch: stored E in plants Both polymers of glucose Structural Polysaccharides Cellulose in plants Chitin in fungi and animals Peptidoglycan in bacteria Lipids: Fats Fatty Acid: Glycerol + Three Fatty Acids (-COOH, carboxyl group) Saturation: no double C=C bonds; the carbons are “saturated” with bonded hydrogen. Unsaturation: one or more C=C double bonds resulting in kinking Longer term E storage than glycogen Phospholipids: two hydrophobic fatty acid tails and a hydrophilic phosphate head Compose the fluid mosaic of membranes Steroids: four carbon rings with various functional groups Proteins Polymers of amino acids: amino group and carboxyl (acid) group on organic molecule 20 types of commonly found, naturally occurring amino acids. Peptide bonds (dehydration) join amino acids into polypeptides. Peptides compose proteins. Primary Structure: amino acid sequence Secondary Structure: helical or sheet shape due to internal hydrogen bonds Tertiary Structure: folding due to H, covalent, and/or ionic bonds resulting in biologically relevant shape Quaternary Structure: two or more polypeptides comprising one protein Nucleic Acids Polymers of nucleotides Nucleotide: phosphate, sugar, N base Sugar is ribose in RNA, deoxyribose in DNA Five types of N bases Cytosine, uracil, adenine, and guanine in RNA Thymine, rather than uracil, in DNA T=A, G=C RNA is single stranded, DNA double helix with bases held together by H bonds ATP Ribose and adenine plus three phosphate groups End phosphate bond is hydrolyzed, giving off E and forming ADP