Introduction to Petrology
advertisement

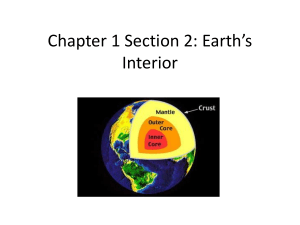
Introduction to Petrology Francis 2014 Introductory Petrology EPSC-212B Don Francis: Documents: Room: F.D.A. 316, donald.francis@mcgill.ca www.eps.mcgill.ca/~c212 1. Igneous: - Elemental abundances, Sun, meteorites, mantle, crust Jan 6 - Units, minerals, phase equilibria Jan 8 - Test on rock forming minerals, review of minerals Jan 9/10 - Phase diagrams for simple mafic systems Jan 13 - Mafic magmas, mantle, and ocean crust Jan 15 - Magmatic textures - Variation diagrams, calc-alkaline versus tholeiitic suites - Mafic Intrusions and cumulate rocks - Volcanic rocks Jan 16/17 Jan 20 Jan 22 Jan 23/24 - Phase diagrams for simple felsic systems Jan 27 - Granitoids and continental crust Jan 29 - Plutonic rocks Jan 30/31 Introductory Petrology EPSC-212B 2. Sedimentary: - Weathering and erosion Feb 3 - Transport and deposition Feb 5 - Igneous test (10 marks), sedimentary structures and textures Feb 6/7 - Siliciclastics Feb 10 - Bio-chemical precipitates I: carbonates Feb 12 - Siliciclastic rocks Feb 13/14 - Bio-chemical precipitates II: dolostones, evaporites, chert, etc. Feb 17 - Depositional environments Feb 19 - Limestones, dolomites, cherts, etc - Mid-Term Test (10 marks) - Sedimentary test (10 marks) Feb 20/21 Feb 26 Feb 27/28 - Cementation and diagenesis Mar 10 - Sedimentary basins and sequence stratigraphy Mar 12 Introductory Petrology EPSC-212B 3. Metamorphic: - Metamorphic minerals and textures Mar 13/14 - Reactions: solid - solid, dehydration and decarbonation Mar 17 - Reactions: mixed volatile, net transfer, exchange Mar 19 - Meta-pelites Mar 20/21 - Meta-pelites Mar 24 - Meta-basites Mar 26 - Meta-basites and meta-carbonates Mar 27/28 - Meta-carbonates Mar 31 - P-T regimes, geothermometry, and geobarometry Apr 2 - Lab review Apr 3/4 - Buffering Apr 7 - Metamorphism and tectonics Apr 9 - Final lab test Apr 10/11 Introductory Petrology EPSC-212B Marking: 2 Lab Spotting Tests (20 marks) Final Lab Exam (20 marks) Mid-Term Exam (10 marks) Final Theory Exam (50 marks) Lectures: Mon & Weds 11:30 - 12:30 am Room: FDA 315 Lab: Thurs 2:30 - 5:30 pm Room: FDA 315 or Fri 2:30 - 5:30 pm Room: FDA 315 Francis, Intro. Petrology EPSC 212, 2014 Texts/References on Reserve in PSE Library and/or my Office Winter, J.D.; 2001: An Introduction to Igneous and Metamorphic Petrology. Prentice Hall, QE461.W735 200 Philpotts, A.R., and Ague, J.J.; 2009: Principles of Igneous and Metamorphic Petrology, Cambridge University Press. Boggs, S.; 2012: Principles of Sedimentology and Stratigraphy. Prentice Hall, New Jersey. QE471 B66. T.A.s: Ryan Libby Gregor Lucic Thomas Maguire Volker Moeller - FDA 346 - FDA 130A - FDA 349 - FDA 346 Francis, Intro. Petrology EPSC 212, 2014 Required Component of Course Outlines Language: In accord with McGill University’s Charter of Students’ Rights, students in this course have the right to submit in English or in French any written work that is to be graded. Integrity: McGill University values academic integrity. Therefore all students must understand the meaning and consequences of cheating, plagiarism and other academic offences under the Code of Student Conduct and Disciplinary Procedures (seewww.mcgill.ca/students/srr/honest/ for more information). Francis, Intro. Petrology EPSC 212, 2013 Igneous Petrology The study of rocks that form by the: crystallization of a cooling melt (“liquid”) or magma Fundamental challenge : to understand high temperature crystal-liquid processes by studying cold solid rocks Diversity of igneous rocks reflects the action of crystal – liquid fractionation processes at high temperature Solid(xyl) K Liquid(liq) glass Elemental partitioning between coexisting solid and liquid Cxyli / Cliqi = Ki followed by the physical separation of solid(s) and liquid 0livine constant temperature (Fe/Mg)oliv / (Fe/Mg)liq ~ 0.3 Natural silicate melts are complex systems with many components and thus melt over a range of temperatures. Because of the high aspect ratios of plagioclase, basalt becomes rigid in the range of 30 to 40% solidification. Note how a cube of solid basalt retains its shape to 70% melting, even as the partial melt drains out of the bottom. 60% basalt cube - % melted 70% 75% Two Kinds of Igneous Rocks: White/Light Granitoids: light or felsic rocks dominated by feldspar and quartz . Constitute the continental crust. Granite Black/Dark Basalt/gabbro: dark or mafic rocks dominated by Fe-Mg silicates, such as olivine, and pyroxenes. Constitute the oceanic crust. Basalt Mantle - Ocean & Continent crust Continental Crust Oceanic Crust Oceanic crust - MORB basalt Continental crust - granite - - e p Composition of the Sun and the Cosmic Abundances of the Elements: As the Sun constitutes 99.98 wt.% of the solar system, the chemical composition of the Sun is also that of the solar system. To determine the proportion of the elements in the Sun, we make use of the energy levels between the electron orbitals of the atoms of the different elements. The electromagnetic spectra of the Sun were noted to contain dark lines in 1802 by Wollaston and later studied by Fraunhofer (early 1800's), indicating adsorption at selective wavelengths or energies. Radiation emerging from the Sun's interior passes though the gas of its photosphere (outermost visible layer), in which the different elements selectively absorb radiation whose wavelength corresponds to the difference in the energy (E = hc/l) levels of its electron orbitals. The intensity of the absorption lines is a measure of the proportion of each element. Solar Spectrum There is a saw-toothed exponential drop off in the abundances of the elements with increasing atomic number, with even numbered elements are always more abundant than adjacent odd numbered elements (Oddo-Harkins rule). The latter presumable reflects the fact that 4He nuclei are the basic component of most element formation reactions in stars. Notice the spike in abundances centered on Fe. Major Elements An analysis of the electromagnetic spectra of the Sun indicates that apart Hydrogen and Helium (98 wt.%), 8 other elements constitute 99 wt.% of the remaining matter (C, N, Ne, O, Mg, Si, Fe). Compared to the Earth's crust, the Sun exhibits a number of important compositional differences. It is depleted in Si, Al, Na, and K, and enriched in Fe and Mg. What has caused these chemical differences between the Sun (~ solar nebula or the solar system as a whole) and the crust of the Earth, and the terrestrial planets in general? This is the story of igneous petrology. Element Sun (Solar System) Earth's Crust Crystal Site O 39.7 46.6 A Fe 27.9 5.0 Y Si 5.5 27.7 T Mg 11.3 2.1 Y Ca 1.3 1.3 X>W Al 1.1 8.1 T~Y Na 0.7 2.8 W>X K 0.1 2.1 W Si/Fe 0.197 5.5 (28 × Sun) K/Fe 0.0036 0.42 (100 × Sun) atomic units atomic units T/Y 0.6 7.3 W/T 0.06 0.18 Dominant Mineral Y2TO4 olivine WT4O8 feldspar Terrestrial Planets basalt or granite crust Fe-Ni Crust represents only ~0.7 wt.% of the Earth basalt or granite crust Fe-Ni opx cpx oliv Allende Chondritic Meteorites = Sun Sun ~ Mantle (~68 wt.%) + Fe-metal core (~31 wt.%) SiO2 + MgO + FeO ~ 90+% The Earth’s upper mantle is similar in composition to the Sun minus enough Fe to form the core. The Earth’s mantle is composed of a rock called peridotite, which consists largely of the minerals olivine and pyroxene Silicon basalt or granite crust Fe-Ni Magnesium Iron Rain drop of the Sun basalt or granite crust feldspar peridotite mantle Iron olivine Chondritic Meteorite + Iron Metal In core Mantle Xenoliths = Sun Mantle Ocean Continent crust crust SiO2 TiO2 Al2O3 MgO FeO CaO Na2O K2O Total 45.2 0.7 3.5 37.5 8.5 3.1 0.6 0.1 99.2 49.4 1.4 15.4 7.6 10.1 12.5 2.6 0.3 99.3 60.3 1.0 15.6 3.9 7.2 5.8 3.2 2.5 99.5 Continental Crust Oceanic Crust Cations normalized to 100 cations Si Ti Al Mg Fe Ca Na K O 38.5 0.5 3.6 47.6 6.0 2.8 0.9 0.1 140.2 46.1 1.0 16.9 10.6 7.9 12.5 4.7 0.5 153.0 56.4 0.7 17.2 5.4 5.6 5.8 5.8 3.0 161.3 Mineralogy (oxygen units, XFe3+ = 0.10) Quartz Feldspar Clinopyroxene Orthopyroxene Olivine Oxides 0.0 13.2 6.7 18.3 59.9 1.8 0.0 57.3 25.7 4.1 9.9 3.0 13.0 64.3 5.9 14.7 0.0 2.0 Oceanic crust - MORB basalt Continental crust - granite - - e p Solid – Liquid Fractionation The diversity of igneous rocks is a reflection of the fact that in a partially melted multi-component system, the composition of the liquid will typically be different than the composition of the solid with which it coexists. Any physical process that separates crystals from liquid, in such a system, will produce a chemical fractionation. liq oliv Partial Melting of the Mantle Solid Source Refractory Solid Restite + Liquid Fertile Mantle Refractory Mantle + Oceanic Crust Cpx-rich Peridotite Lherzolite Olivine-rich Peridotite Harzburgite + Basalt Oceanic Crust Whole = Σ Parts Lever Rule: p/R = x/y Oceanic Crust amount of basalt (P) in fertile mantle = x/(x+y) y x R Crystal Fractionation of Basalt Parent Magma Crystal Cumulate + Residual Magma Plutonic or Intrusive Rocks Mafic Magma Volcanic Rocks Gabbroic Cumulate + Felsic Magma Whole = Σ Parts Lever Rule: e/C = x/y Volcanic rocks approximate the compositions of magmatic liquids. They represent aliquots of liquid that have escaped to the surface. The compositional variation observed in the liquids that the volcanic rocks represent is produced by varying degrees of crystal fractionation of a largely “gabbroic” mineral assemblage that now comprises plutonic intrusions. amount of granite in basalt = x/(x+ y) Cx y Continental Crustal Granitoids Second Stage Melting of Basalt Continental Crust The majority of crustal granitoids are, however, thought to be liquids produced at the eutectic point e by the second stage melting of silicasaturated basaltic/gabbroic mafic crust, consisting largely of pyroxene and plagioclase. e Mantle Ocean Continent crust crust SiO2 TiO2 Al2O3 MgO FeO CaO Na2O K2O Total 45.2 0.7 3.5 37.5 8.5 3.1 0.6 0.1 99.2 49.4 1.4 15.4 7.6 10.1 12.5 2.6 0.3 99.3 60.3 1.0 15.6 3.9 7.2 5.8 3.2 2.5 99.5 Spectrum of Igneous liquids Cations normalized to 100 cations Si Ti Al Mg Fe Ca Na K O 38.5 0.5 3.6 47.6 6.0 2.8 0.9 0.1 140.2 46.1 1.0 16.9 10.6 7.9 12.5 4.7 0.5 153.0 56.4 0.7 17.2 5.4 5.6 5.8 5.8 3.0 161.3 Mineralogy (oxygen units, XFe3+ = 0.10) Quartz Feldspar Clinopyroxene Orthopyroxene Olivine Oxides 0.0 13.2 6.7 18.3 59.9 1.8 0.0 57.3 25.7 4.1 9.9 3.0 13.0 64.3 5.9 14.7 0.0 2.0 Oceanic crust - MORB basalt Continental crust - granite p e