lecture 5
advertisement

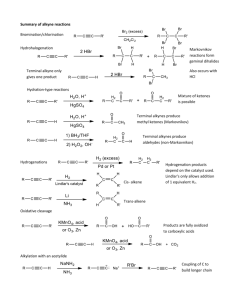
Alkynes .1 Introduction .2 Nomenclature of Alkynes .3 Physical Properties of Alkynes .4 Preparation of Alkynes .5 Reactions of Alkynes Structure Of Alkynes They are unsaturated hydrocarbons – made up of C and H atoms and contain one or more C ≡ C triple bond somewhere in their structures. Their general formula is CnH2n-2. The alkyne triple bond is composed of one σ and two 2 covalent bonds, the triple bond can be terminal or internal. Terminal alkynes have the triple bond at the end of the carbon chain so that a hydrogen atom is directly bonded to a carbon atom of the triple bond. Internal alkynes have a carbon atom bonded to each carbon atom of the triple bond. 2 Structure of Alkynes Acetylene (ethyne): HC CH sp Hybrid orbitals Ground state 2p Exited state sp-hybridized state 2p 2p 2s 2s 1s Promotion of electron 1s sp Hybridization 1s The shape of an sp hybrid obital: Each sp hybrid orbital: 50% s character 50% p character An sp-hybridized carbon atom: 180° Each carbon is sp hybridized with a linear geometry and bond angles of 1800, perpendicular to the two remaining p orbitals. Geometric structure of sp-hybridized C atoms is linear. In the mole. of acetylene, the formation of C-Cσbond : spsp overlap; The formation C-H σ bonds: sp-1s overlap. The formation of two C-C π bonds: 2py-2py overlap and 2pz-2pz overlap. Nomenclature of Alkynes Nomenclature of Alkenes 1. In the IUPAC system, change the –ane ending of the parent alkane name to the suffix –yne. 2. Determine the stem name by selecting the longest possible straight chain containing the C ≡ C triple bond and number the chain to give the triple bond the lower number. 3. Compounds with two triple bonds are named as diynes, those with three are named as triynes and so forth. 3. Designate the position of the C ≡ C triple bond by using the number of the first atom of the double bond Nomenclature of Alkynes Examples: CH3 CH3 C CH CH3 CH CH2 Br CH3 C C CH CH3 IUPAC :2,6-Dimethyl-3-heptyne Common :Isobutylisopropylacetylene HC CH2 5-bromo-2-pentyne Propyne CH3 C C CH2 Pentyne CCH2CH3 1-Butyne 6-Ethyl-4-nonyne 7 Nomenclature of Alkynes 4. Double and triple bonds are considered to have equal priority: thus in a molecule with both a double and triple bond, whichever is close to the end of the chain determines the direction of numbering. 5. In case where double and triple bonds would have the same position number, the double bond takes the lower number. 6. Compounds with both a double and triple bond are named as enynes ( ‘’ene’’ comes before ‘’yne’’). 7. The simplest alkyne, H-CC-H, named in the IUPAC system as ethyne, is more often called acetylene, its common name. 8. The two-carbon alkyl group derived from acetylene is called an ethynyl group. CH3CH CH C 3-Penten-1-yne CH HC CCH2CH CH2 1-penten-4-yne 1 2 F 4 CH F 3 HC 1 2 C 4 7 CH 3 4 5 9 6 1 H3C Br 3 C C 5 8 CH3 5-Bromo-2-pentyne Not 1-Bromo-3-pentyne 6-Ethyl-4-nonyne 4,4-Difluoro-3-methyl-1-butyne 2 H 5 HC 4 C 3 CH2 2 1 CH CH2 Pent-1-en-4-yne double and triple bonds have have the same position number thus ene take lower number Hept-2-en-5-yne or 2-Hepten-5-yne 1 2 HC C 3 C 4 5 6 CH-CH2-CH3 Hex-3-en-1-yne (triple bond closer to the end of chain) Note: An''e'' is dropped from ''ene'' due to it is followed by a vowel Common Nomenclature Of Alkynes The simplest alkyne its common name is acetylene Therefore the common names of alkynes are derived from acetylene ( e.g. Methyl acetylene) Examples: CH3 C CH3 CH CH3 Common : Methyl acetylene H3C C CH CH2 CH3 C C CH CH3 Common : Isobutyisopropylacetylene C Common: Isopropylmethylacetylene Exercise 1. Give the IUPAC and common names of the following compounds: b) a) c) C C H2 C H2 C d) HC C CH C- C(CH3)3 11 Physical Properties of Alkynes Alkynes are non polar compounds. Insoluble in water. Soluble in non polar organic solvents. They are less dense than water. Alkynes have low melting points and boiling points. Melting point and boiling point increase as the number of carbons increases. Terminal alkynes, R-CC-H, are more acidic than other hydrocarbons. 12 Preparation of Alkynes Elimination Reactions 1. Dehydrohalogenation of dihaloalkanes (-2HX) • Dehydrohalogenation is the elimination of (-2HX) from a dihaloalkane in presence strong base Br H H H Br KOH / alcohol Heat Br NaNH2 / NH3 Heat Preparation of Alkynes 2. From reaction of sodium Acetylide with Primary Alkyl Halides R C + CH liq NH 3 Na R C - C Na + + 1/2 H2 R' + Sodium acetylide R C - C Na + + R' X R C C NaX Reactions of Alkenes Addition Reactions Like alkenes, alkynes undergo addition reactions because they contain relatively weak bonds. Two sequential reactions can take place: addition of one equivalent of reagent forms an alkene, which can then add a second equivalent of reagent to yield a product having four new bonds. Reactivity: Alkynes < Alkenes 15 Reactions of Alkenes 1. Addition of hydrogen ( Hydrogenation) Alkynes can be partially reduced to cis-alkenes with H2 in the presence of poisoned catalysts. Lindlar catalyst: Pd/ CaCO3, Pb(Ac)2-quinoline C C + H2 Pd(BASO4) or (NiB) H H Cis- alkene CH3(CH2)3C CH3(CH2)3 H2 C C(CH2)3CH3 Lindlar H catalyst 16 (CH2)3CH3 C H Alkynes can be reduced to trans-alkenes using Na or Li in liquid NH3 (meaning Metal-Ammonia ) H C C + H2 Na or Li / liq. NH3 H Trans -alkene CH3(CH2)3C 17 CH3(CH2)3 H Li or Na C C C(CH2)3CH3 Liq. NH 3 H 78% (CH2)3CH3 Reactions of Alkenes 2. Addition of halogen 3. Addition of hydrogen halide Markovnikov’s rule applies to the addition of HX to vinyl halides X R'C CH HX R'C CH2 X Excess HX Alkenyl halide HX R'C CH3 X Alkyl dihalide 18 4. Addition of water: Hydration Keto-enol tautomerism Transformation of Functional groups: O C C C H(R) Hydroboration—Oxidation Hydroboration—oxidation is a two step reaction sequence that converts an alkyne to a carbonyl compound. 21 Hydroboration—oxidation of an internal alkyne forms a ketone. Hydroboration –oxidation of a terminal alkyne form an aldehyde. 22 Reactions of Alkenes Examples: Four addition reactions of 1-butyne 23 Exercise 1. An alkyne’s name ends with (a) –ane (b) -ene (c) –yne (d) diene 2. An alkyne function has …….. pi bond(s). (a) one (b) two (c) three (d) four 3. Alkynes react with HCl by a mechanism called (a) elimination (b) Markovnikov addition (c) substitution 4. Alkynes react with water in the presence of a catalyst to give (a) a dialcohol (diol) (b) an alkane (c) an enol (d) a dibromide 5. The conversion of alkynes to alkanes is an example of (a) oxidation (b) reduction (c) chlorination (d) dehydration