8. Alkynes: An Introduction to Organic Synthesis
advertisement

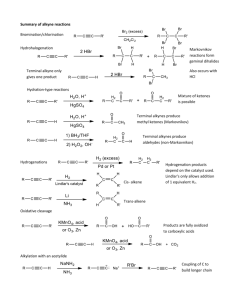
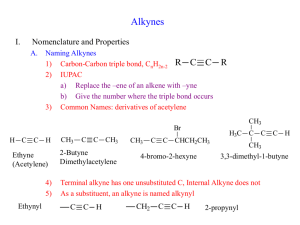
ORGANIC CHEMISTRY 171 Section 201 Alkynes Alkynes • Hydrocarbons that contain carbon-carbon triple bonds • Acetylene is the simplest alkyne. • Our study of alkynes provides nomenclature,physical properties,structure and SPhybridization,preparation and reactions. 3 Alkynes. General Formula CnH2n-2 C2H2 H—C C—H acetylene ethyne C3H4 CH3CCH methylacetylene propyne sp => linear, 180o NOMENCLATURE Naming Alkynes • General hydrocarbon rules apply with “-yne” as a suffix indicating an alkyne. • Numbering of chain with triple bond is set so that the smallest number possible include the triple bond. H3C H2C C C CH2 CH2 CH2 CH2 CH3 3-Nonyne 5 nomenclature: common names: “alkylacetylene” IUPAC: parent chain = longest continuous carbon chain that contains the triple bond. alkane drop –ane add -yne prefix locant for the triple bond, etc. CH3CH2CCCH3 2-pentyne ethylmethylacetylene “terminal” alkynes have the triple bond at the end of the chain: CH3CH2CCH 1-butyne ethylacetylene CH3 HCCCHCH2CH3 3-methyl-1-pentyne sec-butylacetylene Substitutive nomenclature: Similar to alkenes, but with the following differences: 1. The ending "-ene" is replaced with "-yne" H3C C H3C C CH3 4-Methyl-2-pentyne 2. The double bond has a priority over the triple bond when numbered 5 HC 4 C 3 CH 2 2 CH 1 CH2 1-Pentene-4-yne 3. There are no cis-trans-isomers, because the triple bond is linear Enynes • An enyne has a double bond and triple bond. • Number for an Enynes starts at the multiple bond closest to the end (it does not matter wheather it is a double or triple bond) H3C CH2 C C CH2 CH CH2 1-Hepten-4-yne 9 Diyines and Triynes • A compound with two triple bonds is a diyine. – A triyne has three triple bonds. • Number from chain that ends nearest of triple bond. H3C CH2 C C CH2 C CH 1,4-Heptdiyne 10 Alkynes as Substituents Alkynes as substituents are called “alkynyl”. H3C CH2 C C 1-butynyl 11 Cycloalkynes • The smallest cycloalkyne isolated is cyclononyne – the C-C-C bond angle about the triple bond is approximately 156° Physical Properties Similar to alkanes and alkenes of comparable molecular weight and carbon skeletone. Name Ethyne Propyne 1-Butyne Formula HC CH CH3 C CH CH 3 CH 2 C CH Boiling Point (°C) -84 -23 8 Density at 20°C (g/mL) (a gas) (a gas) (a gas) -90 27 40 0.691 0.690 Melting Point (°C) -81 -102 -126 -32 2-Butyne 1-Pentyne CH3 C CCH 3 CH 3 ( CH2 ) 2 C CH 1-Hexyne CH 3 ( CH2 ) 3 C CH -132 71 0.716 1-Octyne CH 3 ( CH2 ) 5 C CH -79 125 0.746 1-Decyne CH 3 ( CH2 ) 7 C CH -36 174 0.766 physical properties: 1-Weakly or non-polar and have no Hbonding. 2-Relatively low m.p. or b.p.but they increase as the molecular weight increase. 3-Water insoluble but soluble in organic solvents. 4-Relatively acidic (have relative acidic character). Acidity • A major difference between the chemistry of alkynes and that of alkenes and alkanes is the acidity of the hydrogen bonded to a triply bonded carbon. Acidity Acetylene reacts with sodium amide to form sodium acetylide – HC CH + pK a 25 Stronger acid NH2 Stronger base HC C- + Weaker base NH3 pK a 38 Weaker acid it can also be converted to its metal salt by reaction with sodium hydride or lithium diisopropylamide (LDA) + Na H– Sodium hydride [ ( CH3 ) 2 CH] 2 N – Li + Lithium diisopropylamide (LDA) Acidity • Water is a stronger acid than acetylene; hydroxide ion is not a strong enough base to convert acetylene to its anion HC CH + OH pK a 25 Weaker Weaker acid base HC C- + Stronger base H2 O pK a 15.7 Stronger acid Keq = 10 -9.3 Electronic Structure of Alkynes • Carbon-carbon triple bond result from • sp hybridized orbital on each C forming a sigma bond at 180º • unhybridized 2pX and 2py orbitals forming a 2p bond • The formed C-C triple bond is shorter and stronger than single or double • Breaking a p bond in acetylene (HC=CH) requires 318 kJ/mole (in ethylene it is 268 kJ/mole) H C C H 18 In other words, in alkynes: 1-One s- and two p- bonds form a triple bond. 2-The valence shell of the atom of carbon has one s-orbital and three p-orbitals. 3- The carbon is bonded by two p-bonds, each of two p-orbitals participates in the formation of this p- bond 4-The remaining one s-orbital and one p-orbital are equally involved in the formation of s-bonds, giving rise to the sphybridization state. So,the presence of sp-hybridized carbons is characteristic for alkynes. s- and 2p-bonds in alkynes p p-bond H C C H s sp p-bond CH HC s-bond Preparation Preparation of Alkynes 1.Elimination Reactions of Dihalides or Dihydrohaloalkenes • Treatment of a 1,2 dihydrohaloalkene with KOH or NaOH (strong Base) produces a two-fold elimination of HX H C C CH2 CH3 Cl H3C 1) 2 NaNH2 2) H3O+ H3C C CH2 CH3 23 dehydrohalogenation of vicinal dihalides H H | | —C—C— | | X X H | + KOH — C = C — | X H | —C=C— | X + NaNH2 — C C — + NaX + NH3 + KX + H2O Preparation of Alkynes: Vicinal Dihalides • Vicinal dihalides are available from addition of bromine or chlorine to an alkene. • Intermediate is a vinyl halide. H C C H Br2 CH2Cl 2 H Br C C Br H 2 KOH + 2 H2O + 2 KBr Ethanol A vicinal dibromide 25 2.Alkylation of Acetylene and Terminal Alkynes – H—C C : SN 2 + R X H—C C—R + The alkylating agent is an alkyl halide, and the reaction is nucleophilic substitution. The nucleophile is sodium acetylide or the sodium salt of a terminal (monosubstituted) alkyne. : X– Example: Alkylation of Acetylene NaNH2 HC HC CH CNa NH3 CH3CH2CH2CH2Br HC C CH2CH2CH2CH3 (70-77%) Example: Alkylation of a Terminal Alkyne (CH3)2CHCH2C CH NaNH2, NH3 (CH3)2CHCH2C CNa CH3Br (CH3)2CHCH2C (81%) C—CH3 Example: Dialkylation of Acetylene H—C C—H 1. NaNH2, NH3 2. CH3CH2Br CH3CH2—C C—H 1. NaNH2, NH3 2. CH3Br CH3CH2—C (81%) C—CH3 Reactions of Alkynes 1. Alkyne Adition Reactions a.Reactions of Alkynes: Addition of HX and X2 • Addition reactions of alkynes are similar to those of alkenes • Intermediate alkene reacts further with excess reagent • Regiospecificity according to Markovnikov HBr HC C CH2 CH3 CH2Cl 2 Br C C CH2 CH3 H H 32 b.Addition of Bromine and Chlorine • Initial addition gives trans intermediate. • Product with excess reagent is tetrahalide. HC C CH 2 CH 3 Br2 CH 2Cl 2 Br H C C Br CH 2 CH 3 Br2 CH 2Cl 2 Br Br H H H C C C C H Br Br H H 33 c.Addition of HX to Alkynes Involves Vinylic Carbocations • Addition of H-X to alkyne should produce a vinylic carbocation intermediate – Secondary vinyl carbocations form less readily than primary alkyl carbocations – Primary vinyl carbocations probably do not form at all C C + H Br H C C + Br- H C C H C C Br Br- HC CH + H Br H C C - + Br H C C Br- H C C Br 34 d.Hydration of Alkynes • Addition of H-OH as in alkenes – Mercury (II) catalyzes Markovinikov HC C CH2 CH3 oriented addition – Hydroborationoxidation gives the non-Markovnikov product HC C CH 2 CH 3 OH C C CH2 CH3 H H HgSO 4, H2SO4 H2O BH3 THF H2O2 OH H C C CH 2 CH 3 HO H 35 Anti- Markinov e.Mercury(II)-Catalyzed Hydration of Alkynes • Mercuric ion (as the sulfate) is a Lewis acid catalyst that promotes addition of water in Markovnikov orientation • The immediate product is a vinylic alcohol, or enol, which spontaneously transforms to a ketone 36 Mechanism of Mercury(II)-Catalyzed Hydration of Alkynes • Addition of Hg(II) to alkyne gives a vinylic cation • Water adds and loses a proton • A proton from aqueous acid replaces Hg(II) 37 Keto-enol Tautomerism • Isomeric compounds that can rapidily interconvert by the movement of a proton are called tautomers and the phenomenon is called tautomerism • Enols rearrange to the isomeric ketone by the rapid transfer of a proton from the hydroxyl to the alkene carbon • The keto form is usually so stable compared to the enol that only the keto form can be observed O OH H Rapid H H H H Enol Tautomer Enol Tautomer (Less favored) (More favored) 38 f.Hydration of Unsymmetrical Alkynes • If the alkyl groups at either end of the C-C triple bond are not the same, both products can form and this is not normally useful • If the triple bond is at the first carbon of the chain (then H is what is attached to one side) this is called a terminal alkyne • Hydration of a terminal always gives the methyl ketone, which is useful 39 g.Hydroboration/Oxidation of Alkynes • BH3 (borane) adds to alkynes to give a vinylic borane • Oxidation with H2O2 produces an enol that converts to the ketone or aldehyde • Process converts alkyne to ketone or aldehyde with orientation opposite to mercuric ion catalyzed hydration 40 Comparison of Hydration of Terminal Alkynes • Hydroboration/oxidation converts terminal alkynes to aldehydes because addition of water is non-Markovnikov • The product from the mercury(II) catalyzed hydration converts terminal alkynes to methyl ketones 41 2.Reduction of Alkynes a.Addition of H2 over a metal catalyst (such as palladium on carbon, Pd/C) converts alkynes to alkanes (complete reduction) b.The addition of the first equivalent of H2 produces an alkene, which is more reactive than the alkyne so the alkene is not observed 42 c.Conversion of Alkynes to cis-Alkenes • Addition of H2 using chemically deactivated palladium on calcium carbonate as a catalyst (the Lindlar catalyst) produces a cis alkene • The two hydrogens add syn (from the same side of the triple bond) 43 d.Conversion of Alkynes to trans-Alkenes • Anhydrous ammonia (NH3) is a liquid below -33 ºC – Alkali metals dissolve in liquid ammonia and function as reducing agents • Alkynes are reduced to trans alkenes with sodium or lithium in liquid ammonia • The reaction involves a radical anion intermediate (see Figure 8-4) 44 3.Oxidative Cleavage of Alkynes • Strong oxidizing reagents (O3 or KMnO4) cleave internal alkynes, producing two carboxylic acids • Terminal alkynes are oxidized to a carboxylic acid and carbon dioxide • Neither process is useful in modern synthesis – were used to elucidate structures because the products indicate the structure of the alkyne precursor 45 4.Alkyne Acidity: Formation of Acetylide Anions • Terminal alkynes are weak Brønsted acids (alkenes and alkanes are much less acidic (pKa ~ 25. See Table 8.1 for comparisons)) • Reaction of strong anhydrous bases with a terminal acetylene produces an acetylide ion • The sp-hydbridization at carbon holds negative charge relatively close to the positive nucleus (see figure 8-5) 46 Alkylation of Acetylide Anions • Acetylide ions can react as nucleophiles as well as bases (see Figure 8-6 for mechanism) • Reaction with a primary alkyl halide produces a hydrocarbon that contains carbons from both partners, providing a general route to larger alkynes 47