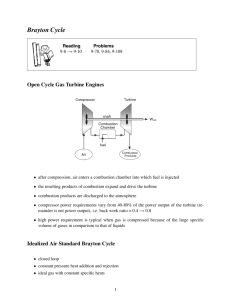
Gas Turbine
advertisement

Applied
Thermodynamics
3. GAS TURBINES AND JET PROPULSION
Introduction:
Gas turbines are prime movers
producing mechanical power
from the heat generated by the
combustion of fuels.
They are used in aircraft, some
automobile units, industrial
installations and small – sized
electrical power generating
units.
A schematic diagram of a
simple gas turbine power plant
is shown below.
This is the open cycle gas
turbine plant.
Working:
Air from atmosphere is
compressed
adiabatically
(idealized) in a compressor
(usually rotary) i.e., Process
1–2.
This compressed air enters the
combustion chamber, where
fuel is injected and undergoes
combustion
at
constant
pressure in process 2–3.
The hot products of
combustion expand in the
turbine
to
the
ambient
pressure in process 3–4 and
the used up exhaust gases are
let out into the surroundings.
The compressor
is usually
coupled to the turbine, so that the
work input required by the
compressor comes from the
turbine.
The turbine produces more work
than what is required by the
compressor, so that there is net
work output available from the
turbine.
Since the products of combustion
cannot be re–used, real gas
turbines work essentially in open
cycles. The p–v and T–s diagrams
of such a plant are shown above.
The compressor
is usually
coupled to the turbine, so that the
work input required by the
compressor comes from the
turbine.
The turbine produces more work
than what is required by the
compressor, so that there is net
work output available from the
turbine.
Since the products of combustion
cannot be re–used, real gas
turbines work essentially in open
cycles. The p–v and T–s diagrams
of such a plant are shown above.
Brayton Cycle:
This is the air–
standard cycle for
the gas turbine plant.
It consists of
two
reversible
adiabatic processes
and two reversible
isobars
(constant
pressure processes).
The p–v and T–s
diagrams
of
a
Brayton Cycle are as
shown.
Process 1 - 2: Reversible
adiabatic compression.
2 – 3: Reversible constant
pressure heat addition.
3 – 4: Reversible adiabatic
expansion.
4 – 1: Reversible constant
pressure heat rejection.
A schematic flow diagram of
this somewhat hypothetical
gas turbine plant is shown
below.
Though this plant works on a closed cycle, each of the four
devices in the plant is a steady–flow device, in the sense that
there is a continuous flow of the working fluid (air) through
each device.
Hence, the steady–flow energy equation is the basis for
analysis, and can be applied to each of the four processes.
Neglecting changes in kinetic and potential energies, the
steady flow energy equation takes the from
Q – W = ∆h = Cp.∆T (Since air is assumed to be an ideal
gas)
Process 1 – 2 is reversible adiabatic, hence Q1-2 = 0
W1-2 = - Cp.∆T = - Cp (T2 – T1): - ve, work input
Work of compression
Wc = |W1 – 2| = Cp (T2-T1)
Process 2–3 is a constant pressure
process
Heat added,
Process 3-4 is again reversible
adiabatic,
+ve work output.
Process 4-1 is also a constant
pressure process
: -ve, i.e., heat is rejected
Heat rejected,
Q2 = |Q4-1| = Cp (T4-T1)
Therefore the cycle efficiency,
Therefore the cycle efficiency,
For isentropic process 1-2,
& for process 3-4,
Since p3 = p2 and p4 = p1
Compression ratio,
Therefore,
Pressure ratio,
Thus it can be seen that, for the same compression ratio,
A closed cycle turbine plant is used in a gas–cooled nuclear
reactor plant, where the source is a high temperature gas
cooled reactor supplying heat from nuclear fission directly
to the working fluid (gas/air).
Comparison between Brayton Cycle and
Otto cycle:For the same
compression
ratio,
and nearly same net
work
output
(represented by the
area inside the p–v
diagram), the Brayton
cycle handles larger
range of volume and
smaller
range
of
pressure than does
the Otto cycle.
A Brayton cycle is not
suitable as the basis for
the working of reciprocating
type of devices (Piston–
Cylinder arrangements).
A reciprocating engine
cannot efficiently handle a
large volume flow of low
pressure gas.
The engine (Cylinder) size
becomes very large and
friction losses become
excessive.
Otto cycle therefore is more
suitable in reciprocating
engines.
However, a Brayton cycle is
more suitable than an Otto
cycle, as a basis for a
turbine plant.
An I.C. engine is exposed to
the highest temperature only
intermittently (for short way
during each cycle), so that
there is time enough for it to
cool.
On the other hand, a gas
turbine, being a steady flow
device,
is
continuously
exposed to the highest
temperature.
Metallurgical considerations,
therefore limit the maximum
temperature that can be
used.
Moreover, in steady flow
machines, it is easier to
transfer heat at constant
pressure than at constant
volume.
Besides, turbines can be
efficiently
handle
large
volume of gas flow.
In view of all these, the
Brayton cycle more suitable
as the basis for the working
of gas turbine plants.
Effect of irreversibility’s in turbine/compressor:
In the ideal Brayton cycle,
compression and expansion of
air are assumed to be reversible
and adiabatic.
In
reality,
however,
irreversibility’s do exist in the
machine
operations,
even
though they may be adiabatic.
Hence the compression and
expansion processes are not
really
constant
entropy
processes.
Entropy tends to be increase (as
per the principle of increase of
entropy).
Effect of irreversibility’s in turbine/compressor:
The T–s diagram of a Brayton
cycle subject to irreversibility’s will
be as shown.
Irreversibility’s result in a reduction
in turbine output by (h4-h4S) and in
an increase in the compressor
input by (h2 – h2S).
Hence the output reduces by the
amount (h4–h4S )+ (h2–h2s).
Though heat input is also reduced
by (h2-h2s), the cycle efficiency is
less than that of an ideal cycle.
The extent of losses due to
irreversibility’s can be expressed
in terms of the turbine and
compressor efficiencies.
Turbine efficiency,
Compressor efficiency,
Methods of improving the efficiency of Brayton
cycle:
Use of regeneration:
The efficiency of the Brayton cycle can be increased by
utilizing part of the energy of exhaust air from the
turbine to preheat the air leaving the compressor, in a
heat exchanger called regenerator.
This reduces the amount of heat supplied Q1 from an
external source, and also the amount of heat rejected
Q2 to an external sink, by an equal amount.
Since Wnet = Q1 - Q2 and both Q1 and Q2 reduce by
equal amounts, there will be no change in the work
output of the cycle.
Heat added
Heat rejected
Turbine output
Compressor input
Q1 = h3 –h2’ = Cp (T3 – T2’)
Q2 = h4’ – h1 = Cp (T4’ – T1)
WT = h3 – h4 = Cp (T3 – T4)
WC = h2 – h1 = Cp (T2 – T1)
Regeneration can be used only if the temperature of air
leaving the turbine at 4 is greater than that of air leaving
the compressor at 2.
In the regenerator, heat is transferred from air leaving the
turbine to air leaving the compressor, thereby raising the
temperature of the latter.
The maximum temperature to which compressed air at 2
can be heated is equal to the temperature of turbine
exhaust at 4.
This, however, is
possible only in an
ideal regenerator.
In reality, T2’<T4.
The ratio of the
actual temperature
rise of compressed
air to the maximum
possible rise is called
effectiveness of the
regenerator.
With a regenerator, since Wnet
remains unchanged, but Q1
reduces, efficiency
η = Wnet/Q1 increases.
This is also evident from the
fact that the mean temperature
of heat addition increases and
the mean temperature of heat
rejection reduces with the use
of the regenerator, and
efficiency is also given by
With regenerator,
In the regenerator,
Heat lost by hot air = Heat gained by cold air
i.e.,
With an ideal regenerator,
therefore,
For a fixed ratio
, the cycle efficiency decreases
with increasing pressure ratio.
In practice, a regenerator is expensive, heavy
and bulky and causes pressure losses, which may
even decrease the cycle efficiency, instead of
increasing it.
2.Multistage compression with inter cooling:
In this arrangement,
compression of air is carried
out in two or more stages with
cooling of the air in between
the stages.
The cooling takes place in a
heat exchanger using some
external
cooling
medium
(water, air etc).
Shown above is a schematic
flow diagram of a gas turbine
plant
with
two-stage
compression
with
inter
cooling.
1-2: first stage compression
(isentropic)
2-3: inter cooling
(heat rejection at
constant pressure)
3-4: second stage
compression (isentropic)
4-3: constant pressure heat
addition
5-6: isentropic expansion
6-1: constant pressure heat
rejection.
Air, after the first stage compression is cooled before it
enters the second stage compressor.
If air is cooled to a temperature equal to the initial
temperature (i.e., if T3=T1), inter cooling is said to be
perfect.
In practice, usually T3 is greater than T1.
Multistage compressor with inter cooling actually
decreases the cycle efficiency.
This is because the average temperature of heat addition
Tadd is less for this cycle 1-2-3-4-5-6 as compared to the
simple Brayton cycle 1-4’-5-6 with the initial state 1. (refer
fig).
Average temperature of heat rejection Trej also reduces,
but only marginally.
Hence efficiency is less for the modified cycle.
However, if a regenerator is also used the heat
added at lower temperature range (4 to 4’) comes
from exhaust gases from the turbine.
So there may be an increase in efficiency
(compared to a simple Brayton cycle) when multi–
stage compression with inter cooling is used in
conjunction with a regenerator.
For a gas turbine plant using 2–stage compression
without a generator,
Q1 = h5 - h4 = Cp(T5 - T4)
WT = h5 - h6 = Cp(T5-T6)
WC = (h2 - h1) + (h4 - h3) = Cp [(T2 - T1) + (T4 - T3)]
WC = (h2 - h1) + (h4 - h3) = Cp [(T2 - T1) + (T4 - T3)]
Wnet = WT – WC
= Cp [(T5 - T6) – {(T 2- T1) + (T4 - T3)}]
3) Multi-Stage expansion with reheating:
Here expansion of working fluid (air) is carried out in 2 or
more stages with heating (called reheating) in between
stages.
The reheating is done in heat exchangers called
Reheaters.
In an idealized cycle, the air is reheated, after each stage
of expansion, to the temperature at the beginning of
expansion.
The schematic flow diagram as well as T-s diagram for a
gas turbine plant where in expansion takes place in two
turbine stages, with reheating in between, are shown.
Multi-Stage expansion with reheating, by itself, does not
lead to any improvement in cycle efficiency. In fact, it only
reduces.
However, this modification together with regeneration may
result in an increase in cycle efficiency.
It can be seen from the T-s diagram that the turbine exhaust
temperature is much higher when multi stage expansion with
reheating is used, as compared to a simple Brayton cycle.
This makes the use of a regenerator more effective and may
lead to a higher efficiency.
Heat added Q1 = (h3 - h2) + (h5 - h4)
= Cp(T3 - T2) + Cp(T5 - T4)
Turbine output WT = (h3 - h4) + (h5 - h6)
= Cp(T3 - T4) + Cp(T5 - T6)
Compressor input WC = h2 - h1 = Cp(T2 - T1)
Ideal Regenerative cycle with inter
cooling and reheat:
Considerable improvement in efficiency is possible by
incorporating all the three modifications simultaneously.
Let us consider a regenerative gas turbine cycle with two
stage compression and a single reheat.
The flow diagram and T-S diagram of such an
arrangement is shown.
Idealized Regenerative Brayton cycle with two stage
compression with inter cooling and also two stage
expansion with reheating – ideal regenerator, equal
pressure ratios for stages, no irreversibilities, perfect inter
cooling and reheating.
Heat added
Q1 = Cp(T5 - T4’) + Cp(T7 - T6)
Turbine output
WT = Cp(T5 - T6) + Cp(T7 - T8)
Compressor input
WC = Cp(T2 - T1) + Cp(T4 - T3)
If perfect inter cooling, no irreversibilities, equal pressure
ratios for stages and ideal regenerator are assumed,
T1=T3, T2=T4=T8’, T5=T7 and T6=T8=T4’
Then, Q1 = Cp(T5 - T4’) + Cp(T7 – T6)
= Cp (T5 - T6) + Cp(T5 - T6)
= (T5 - T6)
Q2 = Cp(T8’ - T1) + Cp(T2 - T3)
= Cp(T2 - T1) + Cp(T2 - T1)
=2 Cp(T2 - T1)
.
It can be seen from this expression that the efficiency
decreases with increasing pressure ratio rp.
Effect of pressure Ratio rp on simple Brayton
Cycle:That means, the more the pressure ratio, the more will
be the efficiency. Temperature T1 (=Tmin) is dependent
on the temperature of surroundings.
Temperature T3 (=Tmax) is limited by metallurgical
considerations and heat resistant characteristics of the
turbine blade material.
For fixed values of Tmin and Tmax, the variation in net
work output, heat added and efficiency with increasing
pressure ratio rp can be explained with the help of a
T-s diagram as shown.
For low pressure ratio, the net work output is small and
the efficiency is also small (Cycle 1 – 2 – 3 - 4).
In the limit, as rp tends 1, efficiency tends to zero (net
work output is zero, but heat added is not zero).
As the pressure ratio increases, the work output
increases and so does the efficiency.
However, there is an upper limit for rp when the
compression ends at Tmax.
As rp approaches this upper limit (rp)max, both net work
output and heat added approach zero values.
However, it can be seen that the mean temperature
heat addition Tadd approaches Tmax, while the mean
temperature of heat rejection approaches Tmin, as rp
comes close to (rp)max.
Hence cycle efficiency, given
by
approaches the Carnot
efficiency i.e.,
rp - (rp)max When the
compression ends at Tmax
i.e., when state point 2 is at
Tmax.
When rp=rpmax,
The variation of net work output Wnet with pressure
ratio rp is shown below.
As rp increases from 1 to (rp)max, Wnet increases from
zero, reaches a maximum at an optimum value of rp
i.e., (rp)opt and with further increase in rp, it reduces
and becomes zero when rp = rpmax
Pressure Ratio for maximum net work output:Wnet= Cp[(T3 - T4) - (T2 - T1)]
T3 = Tmax & T1= Tmin
Condition for maximum Wnet is
i.e.,
It can be seen that,
Maximum net work output
Corresponding to rp = (rp)opt i.e., when Wnet is maximum, cycle
efficiency is
Open Cycle Gas Turbine
Plants:
In practice, a gas turbine plant works
on an open cycle.
Air from atmosphere is first
compressed to a higher pressure in
a rotary compressor, which is usually
run by the turbine itself, before it
enters the combustion chamber.
Fuel is injected into the combustion
chamber
where
it
undergoes
combustion.
The heat released is absorbed by
the products of combustion and the
resulting high temperature; high
pressure products expand in the
turbine producing work output.
The used up combustion
products (exhaust gases)
are let out into the
atmosphere.
In
the
ideal
case,
compression
and
expansion are assumed to
be
isentropic
and
combustion is assumed to
take place at constant
pressure.
The
schematic
flow
diagram and p-v and T-s
diagrams of an open cycle
gas turbine plant are as
shown.
Advantages and disadvantages of closed cycle
over open cycle
Advantages of closed cycle:
1. Higher thermal efficiency
2. Reduced size
3. No contamination
4. Improved heat transmission
5. Improved part load
6. Lesser fluid friction
7. No loss of working medium
8. Greater output and
9. Inexpensive fuel.
Disadvantages of closed cycle:
1. Complexity
2. Large amount of cooling water is required. This
limits its use of stationary installation or marine
use
3. Dependent system
4. The wt of the system pre kW developed is high
comparatively, not economical for moving
vehicles
5. Requires the use of a very large air heater.
Problems:
1. In a Gas turbine installation, the air is taken in at 1
bar and 150C and compressed to 4 bar. The
isentropic of turbine and the compressor are 82%
and 85% respectively. Determine (i) compression
work, (ii) Turbine work, (iii) work ratio, (iv) Th. .
What would be the improvement in the th. if a
regenerator with 75% effectiveness is incorporated
in the cycle. Assume the maximum cycle
temperature to be 8250K.
Solution:
P1 = 1 bar
T3 = 8250K
T1 = 2880K
C = 0.85
P2 = 4 bar
t = 0.82
Case1: Without Regeneration:
Process 1-2s is isentropic i.e.,
T2 s P2
T1 P1
T2 s 288 4
But C T2 s T1
T2 T1
0.4
1.4
r 1
r
428.14 0 K
i.e.,0.85
428.14 288
T2 452.87 0 K
T2 288
Process 3-4s is isentropic
r 1
r
But
0.4
1.4
T4 s P4
1
i.e.,
T4 s 825 554.96
T3 P3
4
T3 T4
825 T4
t
i.e., 0.82
T4 603.57 0 K
T3 T4 s
825 554.96
(i) Compressor work,
WC = CP (T2 – T1)
= 1.005 (452.87 – 288) = 165.69 kJ/kg
(ii) Turbine work,
Wt = CP (T3 – T4)
= 1.005 (825 – 603.57) = 222.54 kJ/kg
(iii) Work ratio = = 0.255
(iv) Thermal Efficiency ,
= 15.2%
Case2: With Regeneration:
We have effectiveness,
T5 T2
T5 452.87
i.e., 0.75
T4 T2
603.57 452.87
T5 = 565.890K
Heat supplied,
QH1 = Q5-3 = CP(T3 – T5)
= 1.005 (825 – 565.89)
= 260.4 kJ/kg
WT WC 56.85 = 0.218
th
1
260.4
QH
Improvement in th due to
regenerator 0.218 0.152
0.152
i.e., 43.6%
= 0.436
2.The maximum and minimum pressure and
temperatures of a gas turbine are 5 bar, 1.2
bar and 1000K and 300K respectively.
Assuming compression and expansion
processes as isentropic, determine the th
(a) when an ideal regenerator is incorporated in
the plant and (b) when the effectiveness of
the above regenerator is 75%.
Solution:
P2 = P3 = 5 bar P1 = P4 = 1.2 bar
T3 = 1000K
T1 = 300K
Process 1-2s is isentropic i.e.,
P2
T2 s
T1
P1
r 1
r
0.4
1.4
5
0
T2 s 300
451.21 K
1.2
Process 3-4s is isentropic i.e.,
T4 s P4
T3 P3
1.2
T4 s 1000
5
0.4
1.4
r 1
r
664.88 0 K
Ideal regenerator: i.e., T5 = T4
Heat supplied = CP (T3 – T5)
= 1.005 [1000 – 664.88] = 336.79 kJ/kg
Wnet = WT – WC = CP (T3 – T4) – CP (T2 – T1)
= 1.005 [1000 – 664.88 – 451.21 + 300] = 183.91
Wnet 183.91
th
QH 336.79
= 0.546 or 54.6%
Regenerator with = 0.75 i.e.,
T5 T2 actual temperature drop
i.e.,
0.75
T4 T2
ideal temperature drop
T5 451.21
0.75
T5 611.46 0 K
664.88 451.21
Heat supplied, QH = CP (T3 – T5)
= 1.005 (1000 – 611.46) = 390.48kJ/kg
Wnet 183.91
th
QH
390.48
= 0.471 or 47.1%
3.Solve the above problem when the adiabatic
efficiencies of the turbine and compressor are 90%
and 85% respectively.
4. A gas turbine plant uses 500kg of air/min, which
enters the compressor at 1 bar, 170C. The
compressor delivery pressure is 4.4 bar. The
products of combustion leaves the combustion
chamber at 6500C and is then expanded in the
turbine to 1 bar. Assuming isentropic efficiency of
compressor to be 75% and that of the turbine to be
85%, calculate (i) mass of the fuel required /min, of
the CV of fuel is 39000KJ/Kg. (ii)net power output
(iii)Overall thermal efficiency of the plant. Assume
CP=1.13KJ/Kg-K,=1.33 for both heating and
expansion.
Solution:
a 500 kg / min 8.33 kg / sec
m
P1 = 1 bar T1 = 2900K P2 = 4.4 bar T3 = 9230K
C = 0.75
t = 0.85 , WN = ? , th ? m f ?
Calorific Value = 39000 kJ/kg
Process 1-2s is isentropic compression
i.e.,
P
1
1
P1V1 P2V2 or T1V1 T2V2 or 1 C
P2
T2 s T1
P1
But
1
T2 s T1
C
T2 T1
T
2904.4
i.e.,
0.4
1.4
443.02 0 K
443.02 290
0.75
T2 494.030 K
T2 290
Process 3-4s is isentropic expansion i.e.,
T4 s P4
T3 P3
But
T
m f ?
We have
1
T4 s 923
4.4
0.32
1.33
639.18 0 K
T3 T4
T3 T4 s
i.e., 0.85
(i)
1
923 T4
T4 681.76 0 K
923 639.18
m a
CV
500
39000
i.e.,
m f C P T3 T2
m f 1.13923 494.03
f = 6.21kg/min
m
(ii) WN = ?
Compressor work, WC = CP (T2 – T1)
= 1.005 (494.03 – 290)
= 205.05 kJ/kg
Turbine work, WT = CP (T3 – T4)
= 1.13 (923 – 681.76)
= 272.6 kJ/kg
WN = WT – WC = 67.55 kJ/kg
a m
f WN
Net work output per minute = m
= (500+6.21) (67.55) = 34194.49 kJ/min
Power output = 569.91 kW
(iii) th = ?
Heat supplied, QH = CP (T3 – T2)
= 1.33 (923 – 494.03)
= 570.53 kJ/kg
WN
67.55
th
QH 570.53
= 0.118 or 11.8%
5. A gas turbine cycle having 2 stage compression
with intercooling in between stages and 2 stages
of expansion with reheating in between the
stages has an overall pressure ratio of 8.
The maximum cycle temperature is 14000K and
the compressor inlet conditions are 1 bar and
270C. The compressors have s of 80% and
turbines have s of 85%.
Assuming that the air is cooled back to its
original temperature after the first stage
compression and gas is reheated back to its
original temperature after 1st stage of expansion,
determine (i) the net work output
(ii) the cycle th.
Solution: T5 = 14000K
T1 = 3000K, P1= 1 bar
C1= 0.8 = C2, t1 = t2 = 0.85 ,T3 = T1 ,T7 = T5
For maximum work output,
P2 P4 P5 P7
P1 P3 P6 P8
P4
P1
P5
8
P8
Intermediate Pr essure ,
P2 P3 P6 P7 2.83 bar
For process 1-2,
But
P2
T2 s T1
P1
1
= 300 (2.83)0.286 = 403.950K
T2 s T1 403.95 300
c1 0.8
T2 429.9 0 K
T2 T1
T2 300
Since
T3 = T1 and P4 P2
P3
P1
We have T4s = T2s = 403.950K
Also since C1 = C2,
T4 = T2 = 429.90K
Compressor work, WC = CP (T2 – T1) + CP (T4 – T3)
= 2 CP (T2 – T1)
= 2 (1.005) (429.9 – 300)
= 261.19 kJ/kg
For process 5 – 6,
T6 s P6
T5 P5
1
1
T6 s 1400
2.83
0.286
1039.720 K
But
T5 T6
t1
T5 T6 s
1400 T6
i.e., 0.85
T6 1093.76 0 K
1400 1039.72
Since T7 = T5 and P5
P7
P6 P8
Since t1 = t2,
, then T8 = T6
T6 = T8 = 1093.760K
Turbine work, Wt = CP (T5 – T6) + CP (T7 – T8)
= 2 CP (T5 – T6)
= 2 (1.005) (1400 – 1093.76)
= 615.54 kJ/kg
WN = WT – WC = 354.35 kJ/kg
th = ?
Heat Supplied,
QH = CP (T5 – T4) + CP (T7 – T6)
= 1.005 (1400 – 429.9 + 1400 – 1093.76)
= 1282.72 kJ/kg
354.35
= 0.276 or 27.6%
th
1282.72
6. Determine the of a gas turbine having two stages
of compression with intercooling and two stages of
expansion with reheat. Given that the pressure ratio
is 4, minimum temperature of the cycle 270C and
maximum temperature of the cycle is 6000C, when t,
C and regenerator are equal to 80%.
( Home work)
7. A two stage gas turbine cycle receives air at 100 kPa
and 150C. The lower stage has a pressure ratio of 3,
while that for the upper stage is 4 for the compressor
as well as the turbine. The temperature rise of the air
compressed in the lower stage is reduced by 80% by
intercooling.
Also,
a
regenerator
of
78%
effectiveness is used. The upper temperature limit of
the cycle is 11000C. The turbine and the compressor
s are 86%. Calculate the mass flow rate required to
produce 6000kW.
Solution:
P1 = 1 bar
T1 =
2880K
P2
3,
P1
P4
4
P3
IC = 0.8
ε = reg = 0.78, T5 = 13730K,
m ? if P = 6000 kW
C1 = C2 = t1 = t2 = 0.86,
Process 1-2s is isentropic compression
T2 s P2
T1 P1
1
T2s = 288 (3)0.286
= 410.750K
But
Also,
C1
T2 s T1
T2 T1
410.75 288
i.e., 0.86
T2 430.730 K
T2 288
IC
T2 T3
T2 T1
430.73 T3
i.e., 0.8
T3 316.54 0 K
430.73 288
Process 3-4s is 2nd stage isentropic compression
1
T4 s P4
T3
P3
T4s = 316.54 (4)0.286 = 470.570K
But
C2
T4 s T3
T4 T3
470.57 316.54
i.e., 0.86
T4 495.64 0 K
T4 316.54
Process 5-6s is 1st stage isentropic expansion
1
P6
T6 s
T5
P
5
1
T6 s 1373
4
0.286
923.59 0 K
But
T5 T6
t1
T5 T6 s
1373 T6
i.e., 0.86
T6 986.510 K
1373 923.59
Process 6-7 is reheating, assume T7 = T5 = 13730K
Process 7-8s is 2nd stage isentropic expansion i.e., T8 s P8
T7 P7
1
T8 s 1373
3
But
T7 T8
t 2
T7 T8 s
1
0.286
1002.79 0 K
1373 T8
i.e., 0.86
T8 1054.630 K
1373 1002.79
Regenerator is used to utilizes the temperature of exhaust gases
i.e.,
Tx T4
Tx 495.64
0K
i
.
e
.,
0
.
78
T
=
931.65
x
T8 T4
1054.63 495.64
We have, Compressor work: WC = CP (T2 – T1) + CP (T4 – T3)
= 1.005 (430.73 – 288 + 495.64 – 316.54)
= 323.44 kJ/kg
Also, Turbine work : WT
= CP (T5 – T6) + CP (T7 – T8)
= 1.005 (1373 – 986.51 + 1373 – 1054.63)
= 708.38 kJ/kg
Net work output, WN = WT - WC
= 384.95 kJ/kg
But, power produced, P m
WN
i.e., 6000 x 1000 = 384.95 x 1000
m
= 15.59 kg/sec
We have, heat supplied, QH = CP (T5 – Tx) + CP (T7 – T6)
= 1.005 (1373 – 931.65 + 1373 – 986.51)
WN
= 831.98 kJ/kg
th
QH
0.463 or 46.3%
8. In a gas turbine plant working on Brayton
cycle, the inlet conditions are 1 bar and 270C.
The compression of air is carried out in two
stages with a pressure ratio of 2.5 for each
stage with intercooling to 270C.
The expansion is carried out in one stage with
a pressure ratio of 6.25.
The maximum temperature in the cycle is
8000C. The of turbine and both compression
stages are 80%. Determine (i) compressor
work, (ii) Turbine work, (iii) Heat supplied, (iv)
cycle , (v) cycle air rate.
Hint: P1 = 1 bar
P4 = P5 = 6.25 bar, P3 = P2 = 2.5 bar
9. The pressure ratio of an open cycle constant pressure gas
turbine is 6. The temperature range of the plant is 150C and
8000C. Calculate (i) th of the plant, (ii) Power developed by
the plant for an air circulation of 5 kg/s, (iii) Air fuel ratio, (iv)
specific fuel consumption. Neglect losses in the system. Use
the following data: for both air and gases: CP 1.005 kJ/kg0K
and = 1.4. Calorific value of the fuel is 42000 kJ/kg, C =
0.85, t = 0.9 and combustion of 0.95.
10. In a G.T. unit with two stage compression and two stage
expansion the gas temperature at entry to both the turbines
are same. The compressors have an intercooler with an
effectiveness of 83%. The working temperature limits are
250C and 10000C, while the pressure limits are 1.02 bar and 7
bar respectively. Assuming that the compression and
expansion processes in the compressors and turbine are
adiabatic with C of 84% and t of 89% for both the stages.
Calculate (i) the air-fuel ratio at the combustion chambers if
the calorific value of the fuel is 38500 kJ/kg, (ii) Power output
in kW for an air flow rate of 1kg/s and (iii) overall cycle .
11. In a reheat gas turbine cycle, comprising one
compressor and two turbine, air is compressed
from 1 bar, 270C to 6 bar. The highest
temperature in the cycle is 9000C. The
expansion in the 1st stage turbine is such that
the work from it just equals the work required
by the compressor. Air is reheated between the
two stages of expansion to 8500C. Assume that
the isentropic s of the compressor, the 1st
stage and the 2nd stage turbines are 85% each
and that the working fluid is air and calculate
the cycle .
Solution:
P1 = 1 bar
T3 = 1173K WT1 = WC
t1 = t2 = 0.85
T1 = 300K
P2 = 6 bar
T5 = 1123K C = 0.85
We have process 1-2 is isentropic i.e.,
T2 S P2
T1 P1
T2 S
1
6
300
1
0.4
1.4
500.5K
T2 S T1
500.5 300
But C
i.e., 0.85
T2 536K
T2 T1
T2 300
Compressor work, WC = CP (T2 – T1)
= 1.005 (536 – 300) = 237 kJ/kg
From data,
WT1 = WC = 237 kJ/kg
= CP (T3 – T4)
T4 = 937 kJ/kg
T3 T4
But t1
T3 T4 S
1173 937
i.e., 0.85
T4 S 895K
1173 T4 S
Process 3-4 is isentropic i.e.,
P4 T4 S 1
P3 T3
895
P4 6
1173
1.4
0.4
2.328 bar
From T-S diagram, intermediate pressure, P4 = P5 = 2.328 bar
Process 5-6s is isentropic in the 2nd stage turbine
T6 S P6
i.e.,
T5 P5
T5 T6
But t 2
T5 T6 S
1
T6 S
1
1123
2.328
0.4
1.4
882 K
1123 T6
i.e., 0.85
T6 918K
1123 882
WT2 = CP (T5 – T6)
= 1.005 (1123 – 918) = 206 kJ/kg
Net work output = WT – WC
= (WT1 + WT2) – WC = 206 kJ/kg
Net heat transfer or heat supplied, Q = QH + QR
Cycle efficiency,
cycle
= CP (T3 – T2) + CP (T5 – T4)
= 640 + 187 = 827 kJ/kg
Wnet
206
25%
Qnet
827
12. In a simple gas turbine unit, the isentropic
discharge temperature of air flowing out of
compressor is 1950C, while the actual
discharge temperature is 2400C. Conditions
of air at the beginning of compression are 1
bar and 170C. If the air-fuel ratio is 75 and net
power output from the unit is 650kW.
Compute (i) isentropic of the compressor
and the turbine and (ii) overall . Calorific
value of the fuel used is 46110 kJ/kg and the
unit consumes 312 kg/hr of fuel. Assume for
gases CP = 1.09 kJ/kg-K and = 1.32 and for
air CP = 1.005 kJ/kg-K and = 1.4.
Solution:
T2S = 195+273 = 468 K
T2 = 240+273 = 513K
T1 = 290K P1=1bar
A/F = 75,
Power output = Wnet = WT – WC = 650kW C = ? T = ?
cycle = ?
CV = 46110 kJ/kg, CPg = 1.09 kJ/kg-k,
g = 1.30, CPa = 1.005 kJ/kg-K, a = 1.4
f 312kg / hr 0.0867 kg / s
m
We have, Compressor Efficiency,
C
T2 S T1
T2 T1
i.e.,
468 290
0.79
513 290
A
Also, ma m f
F
= 75 (0.0867) = 6.503 kg/s
T2 S
Pr essure ratio R
T1
1 468
290
1.4
0.4
5.34
Applying SFEE to the constant pressure heating process 2-3,
m f
CV m a m f CPg T3 T2
0.0867 (46110) = (6.503 + 0.0867) 1.09 (T3 – 513)
T3 = 1069.6K
Also,
1
P4
T4 S
T3
P3
T4S = 712.6K.
g
g
1.321
1.32
T4 S 1069.65.34
Further,
a m
f CPg T3 T4 m
a CPa T2 T1
W net WT WC m
i.e., 650 = (6.503 + 0.0867) 1.09 (1069.6 – T4) – 6.503 (1.005) (513 – 290)
T4 = 776K
Now, Turbine Efficiency,
T3 T4
1069.6 776
T
0.822
T3 T4 S 1069.6 712.6
And,
cycle
Wnet
650
0.163
m f CV 0.086746110
Or
cycle
Wnet
650
650
0.162
m a m f C Pg T3 T2 3997.9
QH