L03_2002
advertisement

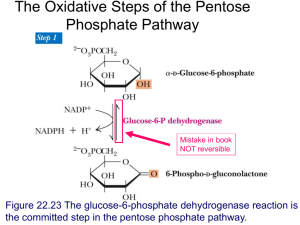
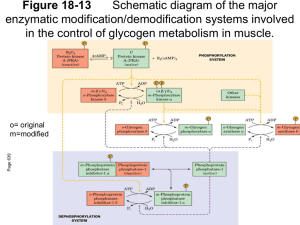
Lecture 3: Phosphorylase (parts of Chapter 15 + Buchbinder et al. 2001) Discussion of paper and talk assignments. Notes: • A PDF of Buchbinder et al. 2001 will be posted on the course web site today. For your professional growth as a scientist (as well as potential extra credit on EXAM 1), you should read this paper. • The course materials will be posted on eRES soon, perhaps as early as this Friday. • The PASSWORD for this course on eRES will be: biochem2 Review: Glycogenolysis requires 3 major enzymes: 1) GLYCOGEN PHOSPHORYLASE (bond cleavage by phosphorolysis) Glycogen(n units) + Pi <---> Glycogen (n-1) + G-1-P inorganic phosphate Glucose-1-phosphate 2) GLYCOGEN DEBRANCHING ENZYME (Fig. 15-6) 3) PHOSPHOGLUCOMUTASE (Fig. 15-7): G-1-P <---> G-1,6-P <---> Glucose-1,6-bisphosphate G-6-P Glucose-6-phosphate G-6-P has several fates (see Fig 15-1). In LIVER, it is hydrolyzed to glucose + Pi by GLUCOSE-6-PHOSPHATASE. Review: Glycogen SYNTHESIS requires 3 major enzymes, and occurs by a SEPARATE PATHWAY from glycogenolysis: 1) UDP-GLUCOSE PYROPHOSPHORYLASE (Fig. 15-9): G-1-P + UTP <---> UDP-glucose (UDPG) + Uridine triphosphate Uridine diphosphate glucose PPi inorganic pyrophosphate 2) GLYCOGEN SYNTHASE (Fig. 15-10): UDPG + Glycogen(n units) <---> UDP + Glycogen(n+1 units) This reaction must be “primed” by GLYCOGENIN 3) GLYCOGEN BRANCHING ENZYME (Fig. 15-11) or AMYLO (1,4--> 1,6) TRANSGLYCOSYLASE. GLYCOGEN PHOSPHORYLASE (Review) • Is a FAST enzyme. • Is a dimer of identical 842-residue subunits. •Removes GLUCOSE UNITS from the NONREDUCING ends of GLYCOGEN. • Catalyzes the CONTROLLING STEP in glycogen breakdown. • Only releases units that are 5 or more from the branch (steric hindrance) • Is regulated by (Fig. 15-5; see Kinemages 14 on the CD with VVP): ALLOSTERIC INTERACTIONS— ATP, G6P & glucose inhibit it; AMP activates it. COVALENT MODIFICATION — phosphorylation at Ser-14 (Phosphorylase A)—more active dephosphorylation (Phosphorylase B )—less active Glycogen phosphorylase is a model for understanding many general principals in biochemistry, including: • The basis of metabolic pathway regulation through regulation of key enzymes • The 3D structural basis of enzyme regulation • Evolution of regulatory mechanisms • Gene duplication and divergence of the ‘isozymes’ • Tissue-specific regulation of isozymes • The structural basis of inherited biochemical disorders (such as McArdle’s disease; see Box 15-2) Gene duplication and divergence is extremely common during evolutionary history Gene duplication Species divergence Species A Gene copy 1 Species B Species A Gene copy 2 Species B The resulting gene duplicates are often expressed in different tissues as different ‘isozymes’ with somewhat different structures and functions. Metabolic pathway regulation through regulation of key enzymes (GP, in this case): Mammals have liverand muscle-specific isozymes of GP Fig. 15-12 3’,5’-Cyclic AMP (cAMP) synthesis and breakdown: Control of GP activity : Most active R form Fig. 15-13 Glycogen Storage Diseases (Box 15-2): Type V: Muscle Phosphorylase Deficiency (McArdle’s Disease) • Inherited (genetic) disease • Causes painful muscle cramps upon exertion • Usually does not appear until early adulthood • Glycogen metabolism in muscle is hindered, so that the muscles can’t get enough fuel for glycolysis • Liver glycogen metabolism is normal HOW could this be? Glycogen Storage Diseases (Box 15-2): Type V: Muscle Phosphorylase Deficiency (McArdle’s Disease) • Inherited (genetic) disease • Causes painful muscle cramps upon exertion • Usually does not appear until early adulthood • Glycogen metabolism in muscle is hindered, so that the muscles can’t get enough fuel for glycolysis • Liver glycogen metabolism is normal HOW could this be? The liver GP isozyme is normal, but the muscle one is not. (There are several different mutations that can cause McArdle’s Disease.) Fig. 15-3a: 3D structure of GP See Kinemage Exercise and Buchbinder et al. now…..