half-reactions
advertisement

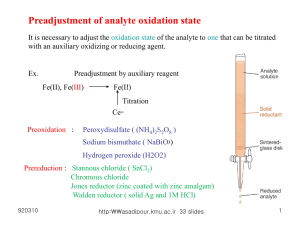
Redox titrations & potentiometry (Mark=3) 920316 http:\asadipour.kmu.ac.ir 81 slides 1 Redox reactions 2Fe3+ + Sn2+ 2Fe2+ + Sn4+ half-reactions: Reduction 2Fe3+ + 2e- 2Fe2+ oxidation 920316 Sn2+ Sn4+ + 2e- http:\asadipour.kmu.ac.ir 81 slides 2 920316 Reduction Half-Reaction E(V) F2(g) + 2e- 2F-(aq) 2.87 Au3+(aq) + 3e- Au(s) 1.50 Cl2(g) + 2 e- 2Cl-(aq) 1.36 Cr2O72-(aq) + 14H+(aq) + 6e- 2Cr3+(aq) + 7H2O 1.33 O2(g) + 4H+ + 4e- 2H2O(l) 1.23 Ag+(aq) + e- Ag(s) 0.80 Fe3+(aq) + e- Fe2+(aq) 0.77 Cu2+(aq) + 2e- Cu(s) 0.34 Sn4+(aq) + 2e- Sn2+(aq) 0.15 2H+(aq) + 2e- H2(g) 0.00 Sn2+(aq) + 2e- Sn(s) -0.14 Ni2+(aq) + 2e- Ni(s) -0.23 Fe2+(aq) + 2e- Fe(s) -0.44 Zn2+(aq) + 2e- Zn(s) -0.76 Al3+(aq) + 3e- Al(s) -1.66 Mg2+(aq) + 2e- Mg(s) -2.37 Li+(aq) + e- Li(s) http:\asadipour.kmu.ac.ir 81 -3.04 slides Red. agent strength increases Ox. agent strength increases Standard Reduction Potentials (strength) 3 Balancing of redox reactions. Under Acidic conditions 1. Identify oxidized and reduced species Write the half reaction for each. 2. Balance the half rxn separately except H & O’s. Balance: Oxygen by H2O Balance: Hydrogen by H+ Balance: Charge by e 3. Multiply each half reaction by a coefficient. There should be the same # of e- in both half-rxn. 4. Add the half-rxn together, the e - should cancel. 920316 http:\asadipour.kmu.ac.ir 81 slides 4 Balancing of redox reactions. Under Basic conditions 1. Identify oxidized and reduced species Write the half reaction for each. 2. Balance the half rxn separately except H & O’s. Balance: Oxygen by H2O Balance: Hydrogen by OHBalance: Charge by e 3. Multiply each half reaction by a coefficient. There should be the same # of e- in both half-rxn. 4. Add the half-rxn together, the e - should cancel. 920316 http:\asadipour.kmu.ac.ir 81 slides 5 Balancing of redox reactions H2O2 (aq) + Cr2O7-2(aq ) Cr 3+ (aq) + O2 (g) Redox reaction ====================================== 1)write 2 half reactions Half Rxn (red): Cr2O7-2 (aq) Cr3+ Half Rxn (oxid): H2O2 (aq) O2 2)Atom balance Cr2O7-2 (aq) 2Cr3+ 920316 H 2O 2 (aq) O2 http:\asadipour.kmu.ac.ir 81 slides 6 Balancing of redox reactions 3)Oxygen balance Half Rxn (red): Cr2O7-2 (aq) 2Cr3+ + 7 H2O Half Rxn (oxi): H2O2 (aq) O2 4)Hydrogen balance Half Rxn (red): 14H+ + Cr2O7-2 (aq) 2Cr3+ + 7 H2O Half Rxn (oxi): H2O2 (aq) O2 + 2H+ 5)Electron balance 6e- + 14H+ + Cr2O7-2 (aq) 2Cr3+ + 7 H2O H2O2 920316 (aq) O2 + 2H+ + 2ehttp:\asadipour.kmu.ac.ir 81 slides 7 Balancing of redox reactions 6) Equalize of produced and consumed electrons 6e- + 14H+ + Cr2O7-2 (aq) 2Cr3+ + 7 H2O ( H2O2 (aq) O2 + 2H+ + 2e- ) x 3 7)Multiply each half reaction 8 H+ + 3H2O2 + Cr2O72- 2Cr+3 + 3O2 + 7H2O 920316 http:\asadipour.kmu.ac.ir 81 slides 8 Balance the redox reactions H+ I2 +S2O32- ⇋ I- +S4O62- OH- I2 +S2O32- ⇋ I- +SO42- 920316 http:\asadipour.kmu.ac.ir 81 slides 9 Preadjustment of analyte oxidation state It is necessary to adjust the oxidation state of the analyte to one that can be titrated with an auxiliary oxidizing or reducing agent. Ex. Preadjustment by auxiliary reagent Fe(II), Fe(III) – 4 Fe(II) Titration Ce4+ Preoxidation : 2– Peroxydisulfate ( (NH4)2S)2O8 ) Sodium bismuthate ( NaBiO 3) Hydrogen peroxide (H2O2) Prereduction : Stannous chloride ( SnCl2) Chromous chloride Jones reductor (zinc coated with zinc amalgam) Walden reductor ( solid Ag and 1M HCl) 920316 http:\asadipour.kmu.ac.ir 81 slides 10 Redox titrations 2Fe3+ + Sn2+ 2Fe2+ + Sn4+ 2) In electrochemical cell. (Potentiometry) 920316 http:\asadipour.kmu.ac.ir 81 slides 1) In solution (visual indicators) 11 920316 http:\asadipour.kmu.ac.ir 81 slides 13 Iodimetry and iodometry • Iodimetry: • a reducing analyte is titrated directly with iodine (to produce I−). • • iodometry : • an oxidizing analyte is added to excess I− to produce iodine, which is then titrated with standard thiosulfate solution. I- + Cu2+→ I2 + Cu+ 920316 I2 + S2O32- → 2I- + S4O62- http:\asadipour.kmu.ac.ir 81 slides 14 Preparation of aqoues solution I31) Iodine only dissolves slightly in water. Its solubility is enhanced by interacting with I- Standardization of Iodin with Arsenious oxide, As2O3 + 3H2O = 2H3AsO3 As4O6 + 6H2O = 4H3AsO3 H3AsO3 + I3– + H2O = H3AsO4 + 3I– + 2H+ 920316 http:\asadipour.kmu.ac.ir 81 slides 15 standard I31) An excellent way to prepare standard I3- is to add a weighed quantity of potassium iodate to a small excess of KI. Then add excess strong acid (giving pH ≈ 1) to produce I3- by quantitative reverse disproportionation: 2) Cu + HNO3 Cu2+ Cu2++4I- 2CUI + I2 920316 http:\asadipour.kmu.ac.ir 81 slides 16 Stability of I2 Solutions • In acidic solutions of I3- are unstable because the excess I− is slowly oxidized by air: • In neutral solutions, oxidation is insignificant in the absence of heat, light, and metal ions. • At pH ≳ 11, triiodide disproportionates to hypoiodous acid (HOI), iodate, and iodide. I2 + OH- ⇌ IO- + I- + H+ 920316 3IO- ⇌ IO3- + 2I- http:\asadipour.kmu.ac.ir 81 slides 17 Iodimetry 920316 http:\asadipour.kmu.ac.ir 81 slides 18 iodometry 920316 http:\asadipour.kmu.ac.ir 81 slides 19 Sodium thiosulfate, Na2S2O3 Thiosulfate ion is a moderate reducing agent that has been widely used to determine oxidizing agents by an indirect procedure that involves iodine as an intermediate. With iodine, thiosulfate ion is oxidized quantitatively to tetrathionate ion according to the half-reaction: 2S2O3 2– S4O6 2– + 2e Eo = 0.08 Ex. Determination of hypochlorite in bleaches [CaCl(OCl)H2O]: OCl– + 2I– + 2H+ Cl– + I2 + H2O (unmeasured excess KI) I2 + 2 S2O3 2– 2I– + S4O6 2– Indicator: soluble starch (-amylose) 920316 http:\asadipour.kmu.ac.ir 81 slides 20 Standardization of thiosulfate solution: Primary standard : potassium iodate (KIO3), K2Cr2O7, KBrO3 Titration reactions: KIO3 + 5KI + 6HCl 3I2 + 6KCl + 3 H2O I2 + 2Na2S2O3 2NaI + Na2S4O6 KIO3 3I2 6Na2S2O3·5H2O 6 Equivalent S2O32- +H+ ⇋ HSO3- +S(s) pH, Microorganisms, Concentration, Cu2+, Sunlight Stabilizer for sodium thiosulfate solution : Na2CO3 Na2S2O3 + H2O + CO2 Na2CO3 + H2S2O3 H2S2O3 H2SO3 + S 920316 http:\asadipour.kmu.ac.ir 81 slides 21 920316 http:\asadipour.kmu.ac.ir 81 slides 22 16-2 Finding the end point A redox indicator is a compound that changes color when it goes from its oxidized to its reduced state. or For ferroin, with E° = 1.147 V we expect the color change to occur in the approximate range 1.088 V to 1.206 V with respect SHE 920316 http:\asadipour.kmu.ac.ir 81 slides 23 920316 http:\asadipour.kmu.ac.ir 81 slides 24 Starch-Iodine Complex Starch is the indicator of choice for those procedures involving iodine because it forms an intense blue colour with iodine. Starch is not a redox indicator; it responds specifically to the presence of I2, not to a change in redox potential. 920316 Structure of the repeating unit of the sugar amylose. http:\asadipour.kmu.ac.ir 81 slides 25 Permanganate titration Oxidation with permanganate : (Reduction of permanaganate) KMnO4 Powerful oxidant that the most widely used. 1) In strongly acidic solutions (1M H2SO4 or HCl, pH 1) MnO4– + 8H+ + 5e = Mn2 + + 4H2 O Eo = 1.51 V KMnO4 is a self-indicator. 2) In feebly acidic, neutral, or alkaline solutions MnO4– + 4H+ + 3e = MnO2 (s) + 2H2 O Eo = 1.695 V 3) In very strongly alkaline solution (2M NaOH) MnO4– + e = MnO42 – 920316 http:\asadipour.kmu.ac.ir 81 slides Eo = 0.558 V 26 Permanganate titration Duration of colour in end point (30 seconds) MnO4– + 3Mn2+ + 2H2O 5MnO2 + 4H+ K=1*1047 Stability of aqoues solution of MnO4MnO4– + 2H2O 4MnO2 (s) + 3O2 (g) +4OH- 920316 http:\asadipour.kmu.ac.ir 81 slides 27 Standardization of KMnO4 solution Potassium permanganate is not primary standard, because traces of MnO2 are invariably present. Standardization by titration of sodium oxalate (primary standard) : 2KMnO4 + 5 Na2(COO)2 + 8H2SO4 = 2MnSO4 + K2SO4 + 5Na2SO4 + 10 CO2 + 8H2O 2KMnO4 920316 5 Na2(COO)2 10 Equivalent http:\asadipour.kmu.ac.ir 81 slides 28 Preparation of 0.1 N potassium permanganate solution KMnO4 is not pure. Distilled water contains traces of organic reducing substances which react slowly with permanganate to form hydrous managnese dioxide. Manganesse dioxide promotes the autodecomposition of permanganate. 1) Dissolve about 3.2 g of KMnO4 (mw=158.04) in 1000ml of water, heat the solution to boiling, and keep slightly below the boiling point for 1 hr. Alternatively , allow the solution to stand at room temperature for 2 or 3 days. 2) Filter the liquid through a sintered-glass filter crucible to remove solid MnO2. 3) Transfer the filtrate to a clean stoppered bottle freed from grease with cleaning mixture and standardize it. 4) Protect the solution from evaporation, dust, and reducing vapors, and keep it in the dark or in diffuse light. 4) If in time managanese dioxide settles out, refilter the solution and restandardize it. 920316 http:\asadipour.kmu.ac.ir 81 slides 29 Applications of permanganometry (1) H2O2 2KMnO4 + 5 H2O2 + 3H2SO4 = 2MnSO4 + K2SO4 + 5O2 + 8H2O (2) NaNO2 2NaNO2 + H2SO4 = Na2SO4 + HNO2 2KMnO4 + 5 HNO2 + 3H2SO4 = 2MnSO4 + K2SO4 + 5HNO3 + 3H2O (3) FeSO4 2KMnO4 + 510 FeSO4 + 8H2SO4 = 2MnSO4 + K2SO4 + 5Fe2(SO4)3 + 8H2O (4) CaO CaO + 2HCl = CaCl2 + H2O CaCl2 + H2C2O4 = CaC2O4 + 2HCl (excess oxalic acid) 2KMnO4 + 5 H2C2O4 + 3H2SO4 = 2MnSO4 + K2SO4 + 10CO2 + 8H2O (back tit) (5) Calcium gluconate [CH2OH(CHOH)4COO]2Ca + 2HCl = CaCl2 + 2CH2OH9CHOH)4COOH (NH4)2C2O4 + CaCl2 = CaC2O4 + 2 NH4Cl CaCl2 920316 + H2SO4 = 2KMnO4 + 5 H2C2O4 H2C2O4 + CaSO4 http:\asadipour.kmu.ac.ir 81 slides 4 + K2SO4 + 10CO2 + 8H2O + 3H2SO4 = 2MnSO 30 Bromatimetry BrO3– + 5Br– + 6H+ 3Br2 + H2O 2I– + Br2 I2 + 2Br– I2 + 2 S2O32– 2I– + S4O62– 920316 http:\asadipour.kmu.ac.ir 81 slides 31 Determining water with the Karl Fisher Reagent The Karl Fisher reaction : I2 + SO2 + 2H2O 2HI + H2SO4 For the determination of small amount of water, Karl Fischer(1935) proposed a reagent prepared as an anhydrous methanolic solution containing iodine, sulfur dioxide and anhydrous pyridine in the mole ratio 1:3:10. The reaction with water involves the following reactions : C5H5N•I2 + C5H5N•SO2 + C5H5N + H2O 2 C5H5N•HI + C5H5N•SO3 C5H5N+•SO3– + CH3OH C5H5N(H)SO4CH3 Pyridinium sulfite can also consume water. C5H5N+•SO3– + H2O C5H5NH+SO4H– It is always advisable to use fresh reagent because of the presence of various side reactions involving iodine. The reagent is stored in a desiccantprotected container. The end point can be detected either by visual( at the end point, the color changes from dark brown to yellow) or electrometric, or photometric (absorbance at 700nm) titration methods. The 81 detection of water by the32 920316 http:\asadipour.kmu.ac.ir coulometric technique with Karl Fischer reagent is popular. slides Potentiometric Methods A.) Introduction: 1.) Potentiometric Methods: based on measurements of the potential of electrochemical cells in the absence of appreciable currents (I →0) 2.) Basic Components: a) reference electrode: gives reference for potential measurement b) indicator electrode: where species of interest is measured c) potential measuring device 920316 http:\asadipour.kmu.ac.ir 81 slides 33 Electrodes and Potentiometry Potential change only dependent on one ½ cell concentrations Reference electrode is fixed or saturated doesn’t change! Ecell=Ecathod-Eanod Anod is conventionally reference electode 0.059 oxi E cathod E cathod – Log n red 2 0.059 oxi E anod E anod – Log n red ] 0 . 222 0 . 05916 log[ Cl ] [ Fe 0.05916 E cell 0.771 log [ Fe 3 ] 1 Fe3+ +e- Fe2+ AgClReference Ag + Cl(s) + e → electrode, Potential of the cell only depends on [Fe2+] & [Fe3+] [Cl-] is constant Unknown solution of [Fe2+] & [Fe3+] 920316 http:\asadipour.kmu.ac.ir 81 slides Pt wire is indicator electrode whose potential responds to [Fe2+]/[Fe3+] 34 Reference Electrodes: (Instead of SHE) Need one electrode of system to act as a reference against which potential measurements can be made relative comparison. Standard hydrogen electrodes are cumbersome - Requires H2 gas and freshly prepared Pt surface Desired Characteristics: a) known or fixed potential b) constant response c) insensitive to composition of solution under study d) obeys Nernest Equation e) reversible E 0.222 0.05916 log[Cl ] > 920316 > http:\asadipour.kmu.ac.ir 81 slides > 35 Reference Electrodes 1.) Silver-Silver Chloride Reference Electrode Eo = +0.222 V Activity of Cl- not 1E(sat,KCl) = +0.197 V 920316 http:\asadipour.kmu.ac.ir 81 slides 36 Reference Electrodes 2.) Saturated Calomel Reference Electrode (S.C.E) Hg 2Cl2 ( s ) 2e 2Hg (l ) 2Cl 920316 Eo = +0.268 V Activity of Cl- not 1E(sat,KCl) = +0.241 V Saturated KCl maintains constant [Cl-] even with some evaporation http:\asadipour.kmu.ac.ir 81 slides 37 Indicator Electrodes 2 Broad Classes of Indicator Electrodes 1) Metal indicator Electrodes - Develop an electric potential in response to a redox reaction at the metal surface 2) Membrane Indicator Electrodes a) Ion-selective Electrodes - Selectively bind one type of ion to a membrane to generate an electric potential b) Molecular Selective Electrode 920316 http:\asadipour.kmu.ac.ir 81 slides 38 1) Metallic Indicator Electrode (3 Main Types) a) Metallic Electrodes of the First Kind b) Metallic Electrodes of the Second Kind c) Metallic Redox Indicators a) Metallic Electrodes of the First Kind i. Involves single reaction ii. Detection of cathione derived from the metal used in the electrode iii. Example: use of copper electrode to detect Cu2+ in solution ½ reaction: Cu2+ + 2eEind gives direct measure of Cu2+: since aCu(s) = 1: or using pCu = -log aCu2+: 920316 Cu (s) Eind = EoCu – (0.0592/2) log aCu(s)/aCu2+ Eind = EoCu – (0.0592/2) log 1/aCu2+ Eind = EoCu – (0.0592/2) pCu http:\asadipour.kmu.ac.ir 81 slides 39 b) Metallic Electrodes of the Second Kind i. Detection of anion derived from the interaction with metal ion (Mn+) from the electrode ii. Anion forms precipitate or stable complex with metal ion (Mn+) iii. Example: Detection of Cl- with Ag electrode ½ reaction: AgCl(s) + e- Eind gives direct measure of Cl-: Ag(s) + Cl- EO = 0.222 V Eind = Eo – (0.0592/1) log aAg(s) aCl-/aAgCl(s) since aAg(s) and aAgCl(s)= 1 & Eo = 0.222 V: Eind = 0.222 – (0.0592/1) log aCl- 920316 http:\asadipour.kmu.ac.ir 81 slides 40 c) Metallic Redox Indicators i. Electrodes made from inert metals (Pt, Au, Pd) ii. Used to detect oxidation/reduction in solution iii. Electrode acts as e- source/sink iv. Example: Detection of Ce3+ with Pt electrode ½ reaction: Ce4+ + eEind responds to Ce4+: 920316 Ce3+ Eind = Eo – (0.0592/1) log aCe3+/aCe4+ http:\asadipour.kmu.ac.ir 81 slides 41 2) Membrane Indicator Electrodes i. electrodes based on determination of Molecules,cations or anions by the selective adsorption of these species to a membrane surface. ii. Ion Selective Electrodes (ISE) or pIon Electrodes are more common. iii. Desired properties of ISE’s 1) minimal solubility – membrane will not dissolve in solution during measurement. – silica, polymers, low solubility inorganic compounds , (AgX) can be used 2) Need some electrical conductivity 3) Selectively binds ion of interest 920316 http:\asadipour.kmu.ac.ir 81 slides 42 Ion-Selective Electrodes Does not involve a redox process Responds Selectively to one ion such as (C+) Contains a thin membrane capable of only binding the desired ion electric potential is generated by a separation of charge E constant 0.05916 log[C ] n ISE + IRE = Combined electrod Constant C+ - Inner solution E2 Constant C+ - membrane E1 C+ - Un known solution E=E1-E2 920316 http:\asadipour.kmu.ac.ir 81 slides 43 pH meter // ISE = (glass membrane ) that preferentially binds H + 1) Combined (glass) electrod === ISE+ Ag/AgCl electrode 2 Electrodes 2) SCE outside electrod 920316 http:\asadipour.kmu.ac.ir 81 slides 48 pH Electrode pH Electrodes 1.) pH Measurement with a Glass Electrode Ag(s)|AgCl(s)|Cl-(aq) || H+(aq,outside) H+(aq,inside) , Cl-(aq)|AgCl(s)|Ag(s) Outer reference electrode Eref1 [H+] outside (analyte solution) Ej E1 [H+] inside Inner reference electrode E2 Eref2 Boundary potential Glassdifference membrane (Eb) = E1 - E2 Selectively binds H+ Eb = c + 0.059 log[H+] Eb = c – 0.059 pH 920316 Electric potential is http:\asadipour.kmu.ac.ir 81 slides generated by [H+] difference across 49 glass membrane Alkali Error H+ not only cation that can bind to glass surface - H+ generally has the strongest binding Get weak binding of Na+, K+, etc Most significant when [H+] or aH+ is low (high pH) - usually pH > 11-12 At low aH+ (high pH), amount of Na+ or K+ binding is significant increases the “apparent” amount of bound H+ 920316 http:\asadipour.kmu.ac.ir 81 slides 56 Acid Error Errors at low pH (Acid error) can give readings that are too high Exact cause not known - usually occurs at pH > 0.5 Glass Electrodes for Other Cations change composition of glass membrane putting Al2O3 or B2O3 in glass enhances binding for ions other than H+ Used to make ISE’s for Na+, Li+, NH4+ 920316 http:\asadipour.kmu.ac.ir 81 slides 57 Redox titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M (1M H2SO4) • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 5ml Before Eq • Fe3+ + e Fe2+ E0=0.771 [Fe 2 ] • E 0.771 - 0.059 Log [Fe3 ] [ Fe2 ] N1V 1 N 2V 2 100 0.5 5 2.5 0.357 N 0.357 M V1 V 2 105 [ Fe3 ] N 2V 2 5 2.5 0.119 N 0.119M V1 V 2 105 0.357 • E 0.771 - 0.059 Log 0.743V 0.119 920316 http:\asadipour.kmu.ac.ir 81 slides 58 Attention 1!!!!!! • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 5ml Before Eq • Fe2+ Fe3+ + e E0=-0.771 [Fe3 ] • E 0.771 - 0.059 Log [Fe 2 ] [ Fe2 ] N1V 1 N 2V 2 100 0.5 5 2.5 0.357 N 0.357 M V1 V 2 105 [ Fe3 ] N 2V 2 5 2.5 0.119 N 0.119M V1 V 2 105 0.119 • E 0.771 - 0.059 Log 0.743V !!!!!!!!!! 0.357 920316 http:\asadipour.kmu.ac.ir 81 slides 59 Attention 2!!!!!! • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 5ml Before Eq • 5Fe2+ 5Fe3+ + 5e E0=-0.771 0.059 [Fe3 ]5 • E 0.771 Log 5 [Fe 2 ]5 920316 [ Fe2 ] N1V 1 N 2V 2 100 0.5 5 2.5 0.357 N 0.357 M V1 V 2 105 [ Fe3 ] N 2V 2 5 2.5 0.119 N 0.119M V1 V 2 105 5 0.059 (0.119) • E 0.771 Log 0.743V !!!!!!!! 5 5 (0.357) http:\asadipour.kmu.ac.ir 81 slides 60 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 10 ml • Fe3+ + e Fe2+ Before Eq E0=0.771 [Fe 2 ] • E 0.771 - 0.059 Log [Fe3 ] [ Fe2 ] N1V 1 N 2V 2 100 0.5 10 2.5 0.227 N 0.045M V1 V 2 110 [ Fe3 ] N 2V 2 10 2.5 0.227 N 0.045M V1 V 2 105 0.045 • E 0.771 - 0.059 Log 0.771V 0.045 920316 http:\asadipour.kmu.ac.ir 81 slides 61 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • Fe3+ + e Fe2+ 15 ml Before Eq E0=0.771 [Fe 2 ] • E 0.771 - 0.059 Log [Fe3 ] [ Fe2 ] N1V 1 N 2V 2 100 0.5 15 2.5 0.109 N 0.109 M V1 V 2 115 [ Fe3 ] N 2V 2 15 2.5 0.326 N 0.326M V1 V 2 115 0.109 • E 0.771 - 0.059 Log 0.799V 0.326 920316 http:\asadipour.kmu.ac.ir 81 slides 62 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 19 ml • Fe3+ + e Fe2+ Before Eq E0=0.771 [Fe 2 ] • E 0.771 - 0.059 Log [Fe3 ] [ Fe2 ] N1V 1 N 2V 2 100 0.5 19 2.5 0.021N 0.021M V1 V 2 119 [ Fe3 ] N 2V 2 19 2.5 0.328 N 0.399M V1 V 2 119 0.021 • E 0.771 - 0.059 Log 0.846V 0.399 920316 http:\asadipour.kmu.ac.ir 81 slides 63 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • Fe3+ + e Fe2+ 19.5 ml Before Eq E0=0.771 [Fe 2 ] • E 0.771 - 0.059 Log [Fe3 ] [ Fe2 ] N1V 1 N 2V 2 100 0.5 19.5 2.5 0.010 N 0.010M V1 V 2 119.5 [ Fe3 ] N 2V 2 19.5 2.5 0.408 N 0.408M V1 V 2 119.5 0.010 • E 0.771 - 0.059 Log 0.866V 0.408 920316 http:\asadipour.kmu.ac.ir 81 slides 64 Titration curve • 1e+Fe3+ Fe2+ 5e+MnO4-+8H+ Mn2+ • E Fe EMnO4 E eq - • E eq E Fe E Fe 20 ml At Eq [Fe 2 ] - 0.059 Log [Fe 3 ] • E eq E MnO4- E MnO 4 0.059 [Mn 2 ] Log 5 [MnO 4 ][H ]8 ×5 2 [Mn ] • 5E eq 5E MnO 4 - 0.059 Log [MnO 4 ][H ]8 2 2 [Fe ][Mn ] • 6E eq E Fe 5E MnO 4 - 0.059 Log [Fe3 ][MnO 4 ][H ]8 920316 http:\asadipour.kmu.ac.ir 81 slides 65 Titration curve 5X • 6E eq E Fe 5E MnO 4 Y [Fe 2 ][Mn 2 ] - 0.059 Log [Fe3 ][MnO 4 ][H ]8 5Y X MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • 6E eq E Fe 5E MnO4 - 0.059 Log E eq 920316 1 [H ]8 /6 E Fe 5E MnO4 0.059 1 Log 8 6 6 [H ] http:\asadipour.kmu.ac.ir 81 slides 66 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 20 ml At Eq • Fe3+ Fe2+ • Mno4-+8H+ Mn2+ nE 0 ox mE 0 red 0.059 1 •E Log (m n) ( m n) [H ]x 5 1.51 1 0.771 1.387V 5 1 920316 http:\asadipour.kmu.ac.ir 81 slides 67 Keq MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • 1e+Fe3+ Fe2+ E Fe E Fe 0.059 [Fe 2 ]5 Log 5 [Fe 3 ]5 E MnO4- E MnO 4 E Fe E MnO 4 5e+MnO4-+8H+ Mn2+ 0.059 [Mn 2 ] Log 5 [MnO 4 ][H ]8 20 ml At Eq EFe EMnO4 0.059 [Fe 2 ]5 0.059 [Mn 2 ] Log E MnO Log 3 5 5 5 [Fe ] [MnO 4 ][H ]8 4 E 920316 Fe 0.059 [Mn 2 ] 0.059 [Fe 2 ]5 Log Log 8 5 5 [Fe 3 ]5 [MnO 4 ][H ] http:\asadipour.kmu.ac.ir 81 slides 68 - Keq At Eq Fe 0.059 [Mn 2 ] 0.059 [Fe 2 ]5 Log Log 8 5 5 [Fe 3 ]5 [MnO 4 ][H ] E MnO 4 E Fe 0.059 [Mn 2 ] 0.059 [Fe 3 ]5 Log Log 8 5 5 [Fe 2 ]5 [MnO 4 ][H ] E MnO 4 E E redox 0.059 [Mn 2 ] [Fe 3 ]5 (Log Log ) 2 5 8 5 [Fe ] [MnO 4 ][H ] E redox 920316 0.059 [Mn 2 ][Fe 3 ]5 Log 5 [MnO 4 ][H ]8 [Fe 2 ]5 http:\asadipour.kmu.ac.ir 81 slides 69 Keq E redox 0.059 [Mn 2 ][Fe 3 ]5 Log 5 [MnO 4 ][H ]8 [Fe 2 ]5 • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 K eq E redox [Mn 2 ][Fe 3 ]5 [MnO 4 ][H ]8 [Fe 2 ]5 - 0.059 Log K eq 5 Log K eq 920316 Log K eq 5E redox 0.059 (5 1)E redox 0.059 http:\asadipour.kmu.ac.ir 81 slides 70 Keq • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • MnO4-+5e-+8H+→ Mn2++ 4H20 • Fe3+ +e → Fe2 + • LogK eq 920316 E0=1.51 E0= 0.771 n=5 n=1 5 1(1.51 - 0.771) 62.5 0.059 http:\asadipour.kmu.ac.ir 81 slides 71 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 20.5 ml After Eq • Mno4-+8H+ Mn2+ 0.059 [Mn 2 ] • E 1.51 Log 5 [MnO 4 ][H ]8 [ Mn 2 ] N1V 1 100 0.5 0.415 N 0.083M V1 V 2 120.5 [ MnO4 ] N 2V 2 N1V 1 20.5 2.5 100 0.5 0.010 N 0.002 M V1 V 2 120.5 0.059 0.083 Log 1.491V 8 5 0.002 1 http:\asadipour.kmu.ac.ir 81 • E 1.51 920316 slides 72 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • Mno4 -+8H+ Mn2+ 21 ml After Eq 0.059 [Mn 2 ] • E 1.51 Log 5 [MnO 4 ][H ]8 [ Mn 2 ] [ MnO4 ] N1V 1 100 0.5 0.413N 0.083M V1 V 2 121 N 2V 2 N1V 1 21 2.5 100 0.5 0.021N 0.004 M V1 V 2 121 0.059 0.083 • E 1.51 Log 1.494V 8 5 0.004 1 920316 http:\asadipour.kmu.ac.ir 81 slides 73 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 22 ml After Eq • Mno4-+8H+ Mn2+ 0.059 [Mn 2 ] • E 1.51 Log 5 [MnO 4 ][H ]8 [ Mn 2 ] [ MnO4 ] N1V 1 100 0.5 0.410 N 0.082M V1 V 2 122 N 2V 2 N1V 1 22 2.5 100 0.5 0.041N 0.008M V1 V 2 122 • E 1.51 920316 74 0.059 0.082 Log 1.498V 8 5 0.008 1 http:\asadipour.kmu.ac.ir 81 slides Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 23 ml After Eq • Mno4-+8H+ Mn2+ 0.059 [Mn 2 ] • E 1.51 Log 5 [MnO 4 ][H ]8 [ Mn 2 ] [ MnO4 ] 920316 N1V 1 100 0.5 0.407 N 0.081M V1 V 2 123 N 2V 2 N1V 1 23 2.5 100 0.5 0.061N 0.012M V1 V 2 123 0.059 0.081 • E 1.51 Log 1.500V 8 5 0.012 1 75 http:\asadipour.kmu.ac.ir 81 slides Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 25 ml After Eq • Mno4-+8H+ Mn2+ 0.059 [Mn 2 ] • E 1.51 Log 5 [MnO 4 ][H ]8 [ Mn 2 ] [ MnO4 ] 920316 N1V 1 100 0.5 0.4 N 0.08M V1 V 2 125 N 2V 2 N1V 1 25 2.5 100 0.5 0.1N 0.02M V1 V 2 125 0.059 0.08 • E 1.51 Log 1.503V 8 5 0.02 1 http:\asadipour.kmu.ac.ir 81 slides 76 Titration curve • 100 ml Fe2+ 0.5 M WITH Mno4- 0.5 M • MnO4-+5Fe2++8H+ Mn2++5Fe3++4H20 • Mno4-+8H+ Mn2+ 30 ml After Eq 0.059 [Mn 2 ] • E 1.51 Log 5 [MnO 4 ][H ]8 [ Mn 2 ] [ MnO4 ] 920316 N1V 1 100 0.5 0.385 N 0.077 M V1 V 2 130 N 2V 2 N1V 1 30 2.5 100 0.5 0.115 N 0.023M V1 V 2 130 0.059 0.077 • E 1.51 Log 1.504V 8 5 0.023 1 http:\asadipour.kmu.ac.ir 81 slides 77 920316 0.743 0.771 0.799 0.846 0.866 1.387 1.491 1.494 1.498 1.5 1.503 1.504 Δ2E/ΔV2 E(v) 5 10 15 19 19.5 20 20.5 21 22 23 25 30 ΔE/ΔV ml of MnO4K Titration curve 0.0056 0.0056 0 0.01175 0.001537 0.04 0.0565 1.042 2.004 0.208 -1.668 0.006 -0.404 0.004 -0.002 0.002 -0.002 0.0015 -0.00025 0.0002 -0.00026 http:\asadipour.kmu.ac.ir 81 slides 78 E Titration curve data 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 Height is related to Keq Not related to concentration 0 920316 10 20 ml of titrant http:\asadipour.kmu.ac.ir 81 slides 30 40 79 Titration curve 1.2 1 ΔE/ΔV 0.8 0.6 0.4 0.2 0 0 -0.2 920316 10 20 30 40 ml of titrant http:\asadipour.kmu.ac.ir 81 slides 80 Titration curve 2.5 2 Δ2E/ΔV2 1.5 1 0.5 0 -0.5 0 10 20 30 40 -1 -1.5 -2 920316 ml of titrant http:\asadipour.kmu.ac.ir 81 slides 81