Chemical Kinetics
advertisement

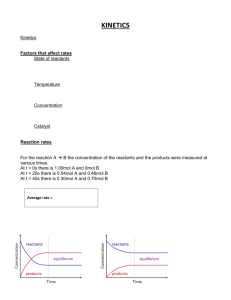
Chemical Kinetics Chapter 16 Kinetics Reaction Rates Factors affecting rate Quantitative rate expressions Determination Factors Models for Rates Reaction Mechanisms Effects of catalysts Rates Change in concentration of a reactant or product per unit time A B Change in conc, A At - A 0 A Change in time, t tt - t0 t 12_292 1.00 Rate = 5.4 x 10-4 mol/L.s [N2O5] (mol/L) .80 Rate = 2.7 x 10-4 mol/L.s .60 .40 .20 400 800 1200 Time (s) 1600 2000 Factors affecting rates Nature of the reactants State of subdivision/surface area Concentration Temperature Catalysts Reactants Complexity Bond strengths Etc. C2 H 4 O3 C2 H 4 O O 2 C2 H 4 O3 C2 H 4 O O 2 C2 H 4 O3 C2 H 4 O O 2 Concentrations as functions of time 2NO2 Time(s) 0 50 100 150 200 250 300 350 400 [NO2] 0.0100 0.0079 0.0065 0.0055 0.0048 0.0043 0.0038 0.0034 0.0031 2NO O2 [NO] 0.0000 0.0021 0.0035 0.0045 0.0052 0.0057 0.0062 0.0066 0.0069 [O2 ] 0.0000 0.0011 0.0018 0.0023 0.0026 0.0029 0.0031 0.0033 0.0035 Graph: Concentration vs. time NO2 NO2 400 - NO2 0 0.0031 - 0.0100 1.725 10 5 M t t 400 - t 0 400 - 0 Concentration vs Time 0.012 Conc.,mol/L 2NO O2 2NO2 [NO2] 0.01 0.008 [NO] 0.006 [O2] 0.004 0.002 0 0 50 100 150 200 250 Time, sec 300 350 400 450 Average Rate Change of concentration in a time interval -[NO2]/t time period(s) –4.20E-05 0 - 50 –2.80E-05 50 - 100 –2.00E-05 100 - 150 –1.40E-05 150 - 200 –1.00E-05 200 - 250 –1.00E-05 250 - 300 –8.00E-06 300 - 350 –6.00E-06 350 - 400 –1.75E-05 0 - 400 Average Rate Slope of line between two points on the graph NO2 NO2 400 - NO2 0 0.0031 - 0.0100 5 M Concentration vs Time 1.725 10 t t 400 - t 0 400 - 0 s 0.012 [NO2] 0.01 Conc.,mol/L 0.008 [NO] 0.006 [O2] 0.004 0.002 0 0 50 100 150 200 250 Time, sec 300 350 400 450 Instantaneous rate Slope of tangent line at a point on the graph y slope of tangent line x NO2 rate t NO2 0.009 M rate @ 100 s t 375 s M rate @ 100 s 2.4 10 s -5 Instantaneous Rate Concentration vs Time 0.012 [NO2] Conc.,mol/L 0.01 0.009 M 0.008 [NO] 0.006 [O2] 0.004 0.002 0 0 50 100 150 200 250 Time, sec 300 350 400 450 375 s 12_291 0.0100 NO2 Concentrations (mol/L) 0.0075 0.0026 [NO 2 ] 0.0006 70s t 110 s 0.005 NO 0.0003 70s 0.0025 O2 50 100 150 200 Time (s) 250 300 350 400 Initial Rate (t = 0) Concentration vs Time 0.012 [NO2] Conc.,mol/L 0.01 0.008 [NO] 0.006 [O2] 0.004 0.002 0 0 50 100 150 200 250 Time, sec 300 350 400 450 Initial rate Slope of tangent line at time 0 (y intercept) y slope of tangent line x NO2 rate t NO2 0.010 M rate @ 0 s t 225 s M rate @ 0 s 4.4 10 s -5 Rate Laws rate kA B m k = m, n = 2NO2 rate = n rate constant order 2NO O2 k[NO2]n Introduction to Rate Laws Reversible chemical reactions Forward: 2NO2 Backward: 2NO O2 Equilibrium: 2NO2 2NO O2 2NO2 2NO O2 Introduction Dominant Reaction: Rate Law: k, k’: n: 2NO2 2NO O2 NO 2 n rate kNO 2 t O 2 n rate k NO2 t specific rate constant order of reactant can be zero, fractional, or negative Method of Initial Rates rate kA B m n Unknown: k, m, n Initial rate: instantaneous rate just after reaction is initiated Initial Rates, NO2 decomposition 2NO2 2NO O2 NO 2 n rate kNO 2 t Experiment 1 2 Initial Conc. [NO2] 0.01 0.02 Rate [O2] Formation 7.1 x 10 -5 2.8 x 10 -4 Order of Reaction General: rate 2 - k 2 NO2 n rate 1 - k1 NO2 n Substituting: 2.8 10 -4 7.1 10 -5 Solution: 4 (2) n ln 4 n - k 2 0.020 n - k1 0.010 n so ln 2 n2 Rate constant NO 2 n rate kNO 2 t Rate 1 7.1 x 10-5 M s-1 k Rate 2 2.8 x 10-4 M s-1 k = = -k[0.01 M]2 0.71 M-1 s-1 = = -k[0.02 M]2 0.70 M-1 s-1 NO2 2 rate law 0.70NO2 t You try H 2 I 2 2HI Experiment Initial Conc. [H2] Initial Conc. [I2] Rate 1 0.0113 0.0011 1.9 x 10-23 2 0.0220 0.0033 1.1 x 10-22 3 0.0550 0.0011 9.3 x 10-23 4 0.0220 0.0056 1.9 x 10-22 O2 + 2 NO 2NO2 Overall Order I rate kH 2SeO3 H Sum: 1 = + 6 2 Overall order of reaction: + 6 2 3 3 Types Differential: Rate dependence on concentration NO 2 n rate kNO 2 t O 2 n rate k NO2 t Integrated: Concentration dependence on time First Order Reactions For aA products Differential: A rate kA t Integrated: ln At - kt ln A0 A 0 ln A t kt Half-life, first order reactions Integrated law: A 0 ln A t Half-life: Half of initial reacted [A]t = ½[A]0 Independent of [A]0 t1 t1 2 2 kt ln2 k 0.693 k Second Order Reactions For aA products Differential: Integrated: A 2 rate kA t 1 1 kt At A0 1 1 kt At A0 Half-life, second order reactions Integrated law: 1 1 kt At A0 Half-life: Half of initial reacted [A]t = ½[A]0 Inversely proportional to [A]0 t1 2 1 kA0 Zero Order Reactions For aA products Differential: A 0 rate kA k t At - kt A0 Integrated: At A0 - kt Graphical Method First order ln At - kt ln A0 Second order 1 1 kt At A0 Zero order At Straight line - kt A0 y mx b First order ln[A]0 ln At - kt ln A0 y mx b slope = -k ln[A] Plot: ln[A] vs. time time Second order 1 1 kt At A0 y mx b Plot: 1 vs. time [A] slope = k 1 [A] 1 [A]o time Zero order At - kt A0 y mx b [A]0 slope = -k [A] Plot: [A] vs. time time Summary Conditions set so dominant forward reaction Differential Rate Laws rate as a function of concentration method of initial rates Integrated Rate Laws concentration as a function of time graphical method Experimental data collection Rate law types can be interconverted Reaction Mechanism Chemical equation: Summary Mechanism: Series of elementary steps Elementary Steps: Reactions with rate laws from molecularity Molecularity: Number of species that must collide to produce reaction Reaction Mechanism Proposed elementary steps must satisfy conditions: — reasonable reactions — sum of steps = overall balanced reaction — mechanism rate law = experimental rate law Intermediates — appear in steps — produced in one step — used in subsequent — not in overall equation Rate-determining step In a multi-step process: SLOWEST step Determines overall reaction rate “Bottleneck” Model for Kinetics Collision Theory rate determined by particle collisions collision frequency and energy Transition State Theory how reactants convert to products Collision Theory (Bimolecular Collsions) rate Z f a p Z: fa : P: no. of bimolecular collisions per second fraction with Ea fraction with correct orientation Ea: activation energy Arrhenius Equation k Ea RT Ae k: rate constant Ea: activation energy (minimum required) T:absolute temperature R: universal gas constant A: orientation factor Energy & orientation requirements for reaction Hydrolysis of an ester Transition State Theory Ea and internal energy: Bonds breaking and forming Atoms rearranging “Transition State” Unstable intermediate At point of highest energy forward reaction reverse reaction exothermic reaction I- + CH3Cl Cl- + CH3I Catalysts Speed reaction Are not consumed Alternative pathway for reaction with lower Ea Types Homogeneous Heterogeneous Enzymes are biological catalysts Number of collisions with a given energy Effective collisions (uncatalyzed) Number of collisions with a given energy 12_304 Ea (catalyzed ) E a (uncatalyzed ) Energy (a) Effective collisions (catalyzed) Energy (b) Adsorption, activation, reaction, desorption