kompendium nitrogen dalam tanah
advertisement

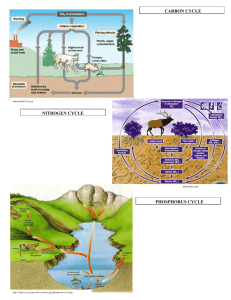
NITROGEN DALAM TANAH bahan kajian MK. Dasar Ilmu Tanah diabstraksikan : Soemarno, jursn tnhfpub 2012. Nitrat (NO3-) esential bagi pertumbuhan tanaman Protein dalam sel tanaman Penyerapan oleh akar tanaman Nitrat NO3Dalam tanah 2 Nitrat di-daur-ulang oleh mikroba Protein binatang N-organik dalam tanah Ammonifikasi Protein tumbuhan Penyerapan oleh akar tanaman Ammonium NH4+ Nitrifikasi Nitrit NO2Nitrifikasi Nitrat tanah NO3- 3 SIKLUS NITROGEN (diunduh dari: ecoplexity.org) 4 5 SIKLUS NITROGEN (diunduh dari: http://www.uwyo.edu/virtual_edge/lab24/nitrogencycle.html ) Bentuk-bentuk Nitrogen • • • • • • • Urea CO(NH2)2 Ammonia NH3 (gas) Ammonium NH4+ Nitrat NO3 Nitrit NO2 Dinitrogen di atmosfir N2 N-Organik Cadangan Nitrogen Dunia Nitrogen Reservoir Atmosphere Metric tons nitrogen 3.9*1015 Actively cycled Ocean soluble salts Biomass 6.9*1011 5.2*108 Yes Yes Land organic matter Biota 1.1*1011 2.5*1010 Slow Yes No 7 Peranan Nitrogen • Tumbuhan dan bakteri menggunakan nitorgen dalam bentuk NH4+ atau NO3• It serves as an electron acceptor in anaerobic environment • Nitrogen is often the most limiting nutrient in soil and water. Nitrogen merupakan unsur kunci bagi: • Asam-asam amino • Asam-asam nukleat (purine, pyrimidine) • cell wall components of bacteria (NAM). Siklus Nitrogen • • • • • Ammonifikasi / Mineralisasi Immobilisasi Fiksasi nitrogen Nitrifikasi Denitrifikasi The soil nitrogen cycle (Adapted from Hofman and Van Cleemput, 2004) Diunduh dari: http://www.fertilizer.org/ifa/HomePage/SUSTAINABILITY/Climate-change/Nitrogen-cycle.html Bentuk organik N-tanah Over 90% of the nitrogen N in the surface layer of most soils occurs in organic forms, with most of the remainder being present as NH4- whichis held within the lattice structures of clayminerals. The surface layerof most cultivated soils contains between0.06 and 0.3% N. Peat soils have high N contents to 3.5%. Plant remains and other debris contribute nitrogen N in the form of: Amino acids Amino acids exist in soil in several different forms, like: As free amino acids in the soil solution in soil micropores As amino acids, peptides or proteins bound to clay minerals on external surfaces on internal surfaces As amino acids, peptides or proteins bound to humic colloids H-bonding and van der Waals' forces in covalent linkage as quinoid-amino acid complexes As mucoproteins As a muramic acid Diunduh dari: http://www.humintech.com/001/articles/article_definition_of_soil_organic_matter9.html BENTUK ORGANIK N-TANAH Amino sugars Amino sugars occur as structal components of a broad group of substances, the mucopolysaccharides and they have been found in combination with mucopeptides and mucoproteins. Some of the amino sugar material in soil may exist in the form of an alkaliinsoluble polysaccharide referred to as chitin. Generally the amino sugars in soil are of microbial origin .From 5 to 10%of the N in the surface layer of most soils can be accounted for in N-containing carbohydrates or amino sugars. Diunduh dari: http://www.humintech.com/001/articles/article_definition_of_soil_organic_matter9.html BENTUK ORGANIK N-TANAH Nucleic Acids Nucleic acids, which occur in the cells of all living organisms, consist of individual mononucleotide units (base-sugar-phosphate) joined by a phosphoricacid ester linkage through the sugar.Two types: ribonucleic acid (RNA) anddeoxyribonucleic acid (DNA). They have pentose sugar (ribose or deoxyribose),the purine: adenine, guanine and the pyrimidine: cytosine, thymine.RNA contains also the uracil. The N in purine and pyrimidine bases is usually considered to account forless than 1% of the total soil N. Small amounts of N are extrcted from soil in the form of glycerophosphatides, amines, vitamins, pesticide and pesticide degradation products. Diunduh dari: http://www.humintech.com/001/articles/article_definition_of_soil_organic_matter9.html Transformasi N-tanah A key feature of the internal cycle is the biological turnover of N betweenmineral and organic forms through the opposing processes of mineralizationand immobilization. The latter leads to incorporation of N into microbial tissues. Whereas much of this newly immobilized N is recycled through mineralization, some is converted to stable humus forms. The overall reaction leading to incorporation of inorganic forms of N intostable humus forms is depicted on the picture. Thus the decay of plant and animal residues by microorganisms results in theformation of mineral forms of N (NH4+ and NO3-) and assimilation of part ofthe C into microbial tissue (reaction A). Part of the native humus undergoes a similar fate (reaction B). Subsequent turnover through mineralization-immobilization leads to incorporation of N into stable humus forms (reaction C). Stabilization of N may also occur through the reaction of partial decay products of lignin with nitrogenous constituents (raection D). Except under unusual circumstances, both mineralization and immobilizationalways function in soil, but in opposite direction. Diunduh dari: http://www.humintech.com/001/articles/article_definition_of_soil_organic_matter9.html Reaksi kimia ammonia dan nitrit dengan bahan organik The fate of mineral forms of N in soil is determined to some extent bynonbiological reactions involving NH4+, NH3and NO2- as depicted in fig. In addition to NH4+ fixation by clay minerals (reaction A), NH3 and NO2- react chemically with organic matter to form stable organic N complexes(reaction B and C). The chemical interaction of NO2- with organic matter may lead to the generation of N gases. Although both types of reactions can proceed over a wide pH range, fixation of NH3+ is favored by a high pH (>7.0). In contrast, NO2- -organic matter interactions occur most readily under highly acidic conditions (pH of 5.0 to 5.5 or below). Diunduh dari: http://www.humintech.com/001/articles/article_definition_of_soil_organic_matter9.html C/N RASIO For surface soils, and for the top layer of lake and marine sediments, the ratio generally falls within well-defined limits, usually from about10 to 12. In most soils, the C/N ratio decreases with increasing depth, often attaining values less than 5.0. Native humus would be expected to have a lower C/N ratio than most undecayedplant residues for following reasons. The decay of organic residues by soilorganisms leads to incorporation of part of the C into microbial tissue with the remainder being liberated as CO2. As a general rule, about one-third of the applied C in fresh residues will remain in the soil after the first few months of decomposition. The decay process is accompanied by conversion of organic form of N to NH3 and NO3- and soil microorganisms utilize partof this N for synthesis of new cells. The gradual transformation of plantraw material into stable organic matter (humus) leads to the establishmentof reasonably consistent relationship between C and N. Other factors which may be involved in narrowing of the C/N ratio include chemical fixation of NH3 or amines by ligninlike substances. The C/N ratio of virigin soils formed under grass vegetation is normally lower than for soils formed under forest vegetation, and for the latter,the C/N ratio of the humus layers is usually higher than for the mineral soil proper. Also the C/N ratio of a well-decomposed muck soil is lower than for a fibrous peat. As a general rule it can be said that conditions which encourage decompositionof organic matter result in narrowing of the C/N ratio. The ratio nearly alwaysnarrows sharply with depth in the profile; for certain subsurface soils C/Nratios lower than 5 are not uncommon. Diunduh dari: http://www.humintech.com/001/articles/article_definition_of_soil_organic_matter9.html SIKLUS N N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 Ammonifikasi • Nitrogen memasuki tanah melalui proses dekomposisi protein dalam bahan organik tanah Amino acids + 11/2O2 CO2 + H2O + NH3 + 736kJ • This process liberates a lot of energy which can be used by the saprotrophic microbes Nitrifikasi • Nitrifikasi melibatkan dua proses oksidasi • The ammonia produced by ammonification is an energy rich substrate for Nitrosomas bacteria They oxidise it to nitrite: NH3 + 11/2O2 NO2- + H2O + 276kJ This in turn provides a substrate for Nitrobacter bacteria oxidise the nitrite to nitrate: NO3- + 1/2O2 NO3- + 73 kJ • This energy is the only source of energy for these prokaryotes • Proses ini bersifat chemo-autotrophs Fiksasi nitrogen dari atmosfer • Arus listrik • Lightning provides sufficient energy to split the nitrogen atoms of nitrogen gas, • Membentuk oksida nitrogen: NOx dan NO2 21 Ammonifikasi atau Mineralisasi N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 22 Mineralisasi atau Ammonifikasi • Decomposers: earthworms, termites, slugs, snails, bacteria, and fungi • Uses extracellular enzymes initiate degradation of plant polymers • Microorganisms uses: • Proteases, lysozymes, nucleases to degrade nitrogen containing molecules 23 • Plants die or bacterial cells lyse release of organic nitrogen • Organic nitrogen is converted to inorganic nitrogen (NH3) • When pH<7.5, converted rapidly to NH4 • Example: Urea NH3 + 2 CO2 24 Immobilisasi • The opposite of mineralization • Happens when nitrogen is limiting in the environment • Nitrogen limitation is governed by C/N ratio • C/N typical for soil microbial biomass is 20 • C/N < 20 Mineralization 25 • C/N > 20 Immobilization Fiksasi Nitrogen N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 26 Fiksasi Nitrogen • Energy intensive process : • N2 + 8H+ + 8e- + 16 ATP = 2NH3 + H2 + 16ADP + 16 Pi • Performed only by selected bacteria and actinomycetes • Performed in nitrogen fixing crops (ex: soybeans) 27 Fiksasi N Biologis Treatments Yield / g Oats No nitrate & sterile soil Peas 0.6 0.8 Nitrate added & sterile soil 12.0 12.9 No nitrate & non-sterile soil 0.7 16.4 11.6 15.3 Nitrate added & non-sterile soil 28 Bintil Akar Alafalfa (Medicago sativa) USDA - ARS University of Sydney Organisme prokaryotik mampu fiksasi N • These organisms possess the nif gene complex which make the proteins, such as nitrogenase enzyme, used in nitrogen fixation • Nitrogenase is a metalloprotein, protein subunits being combined with an iron, sulphur and molybdenum complex • The reaction involves splitting nitrogen gas molecules and adding hydrogen to make ammonia N2 2N 2N + 8H+ NH3 + H2 - 669 kJ + 54 kJ • This is extremely energy expensive requiring 16 ATP molecules for each nitrogen molecule fixed • The microbes that can fix nitrogen need a good supply of energy Pem-fiksasi Nitrogen • Cyanobacteria are nitrogen fixers that also fix carbon (these are photosynthetic) • Rhizobium bacteria are mutualistic with certain plant species e.g. Legumes • They grow in root nodules • Azotobacter are bacteria associated with the rooting zone (the rhizosphere) of plants in grasslands 31 Mikroorganisme Fiksasi N • • • • • Azobacter Beijerinckia Azospirillum Clostridium Cyanobacteria • Require the enzyme nitrogenase • Inhibited by oxygen • Inhibited by ammonia (end product) 32 Laju Fiksasi Nitrogen N2 fixing system Rhizobium-legume Nitrogen Fixation (kg N/hect/year) 200-300 Cyanobacteria- moss 30-40 Rhizosphere associations Free- living 2-25 1-2 33 34 Aplikasi ke Lahan Basah • • • • • Occur in overlying waters Aerobic soil Anaerobic soil Oxidized rhizosphere Leaf or stem surfaces of plants 35 Fiksasi oleh Bacteri • Proses ini dapat terjadi dalam tanah • Is absent from low pH peat of northern bogs • Cyanobacteria ditemukan pada tanah-tanah tergenang Nitrifikasi N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 37 Nitrifikasi Dua tahapan reaksi yang terjadi bersamaan : • 1rst step catalyzed by Nitrosomonas 2 NH4+ + 3 O2 2 NO2- +2 H2O+ 4 H+ • 2nd step catalyzed by Nitrobacter • 2 NO2 + O2 2 NO3 Nitrifikasi • pH Optimal pH : 6.6-8.0 • Kalau pH < 6.0 laju reaksinya lambat • Kalau pH < 4.5 Reaksinya terhambat In which type of wetlands do you thing Nitrification occurs? Denitrifikasi N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 40 Denitrifikasi • Removes a limiting nutrient from the environment • 4NO3- + C6H12O6 2N2 + 6 H20 • Dihambat oleh O2 • Tidak dihambat oleh amonia • Reaksi mikrobiologis • Nitrat merupakan aseptor elektron 41 PERANAN NH4 DALAM SIKLUS NITROGEN 43 Surface water Oxidized layer Reduced soil layer Low [NH4] Biodegradation Slow Diffusion C/N <20 C/N >20 [NH4] HIGH Surface water nitrification Low [NH4] Oxidized layer Reduced soil layer Slow Diffusion [NH4] HIGH [NO3] high N2 Surface water Oxidized layer Reduced soil layer [NO3] high Leaching [NO3] Low Denitrification Dampak Kegiatan Manusia Atmospheric Nitrogen Atmospheric fixation Out gassin g Industrial fixation Plant protein Biological fixation Soil organic nitrogen Ammonium NH4+ Nitrate NO347 Sumber: © 2008 Paul Billiet ODWS Fiksasi N secara Industri • Proses Haber-Bosch N2 + 3H2 2NH3 - 92kJ • Proses Haber menggunakan katalis besi • Suhu tinggi (500°C) • Tekanan tinggi (250 atm) • The energy require comes from burning fossil fuels (coal, gas or oil) • Hydrogen is produced from natural gas (methane) or other hydrocarbon Sumber-sumber Fiksasi Nitrogen Sources of fixed nitrogen Production / M tonnes a-1 Biological 175 Industrial 50 Internal Combustion 20 Atmospheric 10 Sumber; © 2008 Paul Billiet ODWS Eutrofikasi • Pengkayaan hara ekosistem perairan • Nitrat dan ammonia mudah larut dalam air • Kedua bentuk nitrogen tersebut mudah tercuci dari tanah • Sehingga tanah –tanah ini cenderung defisien nitrogen • When fertiliser is added to these soils it too will be washed out into water bodies • There algae benefit from the extra nitrogen • This leads to a serious form of water pollution Eutrophication is the enrichment of an ecosystem with chemical nutrients, typically compounds containing nitrogen, phosphorus, or both. Source: http://www.pbl.nl/en/index.html Diunduh dari: http://biogenicsilica.blogspot.com/2010/08/eutrophication.html Bahaya Eutrofikasi . Many lakes around the world have been effected by discharges of nutrients directly into them. In severe cases this can lead to the process of eutrophication. Inputs of nutrients from sources such excessive over use chemical fertilizers on agricultural land can lead to accelerated growth of algae creating massive blooms. Some of these blooms can be are toxic. Diunduh dari: http://sciencebitz.com/?page_id=597 Kerusakan Ekosistem Danau Berkurangnya oksigen terlarut Peningkatan kandungan nitrit NO3- NO2- Death/emigration of freshwater fauna Methaemoglobinaemia in infants Stomach cancer link (WHO limit for nitrates 10mg dm-3) Masa depan Industri Fiksasi N • Food production relies heavily upon synthetic fertilisers made by consuming a lot of fossil energy • Produksi pangan menjadi lebih mahal • Nitrogen fixing microbes, using an enzyme system, do the same process at standard temperatures and pressures essentially using solar energy • Jawab: Rekayasa genetik fiksasi N biologis? 54 Memperbaiki aplikasi nitrogen • Kebutuhan pupuk sintetis dapat dikurnagi dengan perbaikan teknologi budidaya tanaman • Menghindari penggunaan pupuk mudah larut pada tanah berpasir untuk mencegah pencucian hara • Rotating crops permits the soil to recover from nitrogen hungry crops (e.g. wheat) • Adding a nitrogen fixing crop into the rotation cycle • Ploughing aerates the soil and reduces denitrification • Draining water logged soil also helps reduce denitrification Denitrifikasi • Nitrat dan nitrit dapat digunakan sebagai sumber oksigen oleh bakteri Pseudomonas • Favourable conditions: Cold waterlogged (anaerobic) soils 2NO3- 3O2 + N2providing up to 2385kJ 2NO2- 2O2 + N2 • The liberated oxygen is used as an electron acceptor in the processes that oxidise organic molecules, such as glucose • Mikroba ini bersifat “heterotroph” Beberapa Hal Penting • Pemupukan nitrogen membantu pertumbuhan tanaman • Keberadaan mikroba tanah bermanfaat bagi pertumbuhan tanaman • Bakteri bintil bersimbiosis dnegan kaar tanamna legume memfiksasi nitrogen HASIL-HASIL PENELITIAN . Nitrogen deposition and climate effects on soil nitrogen availability: Influences of habitat type and soil characteristics E.C. Rowe , B.A. Emmett , Z.L. Frogbrook , D.A. Robinson , S. Hughes Science of The Total Environment. Volume 434, 15 September 2012, Pages 62–70 The amount of plant-available nitrogen (N) in soil is an important indicator of eutrophication of semi-natural habitats, but previous studies have shown contrasting effects of N deposition on mineralisable N in different habitats. The stock of readily mineralisable N (Nrm) was measured in 665 locations across Britain from a range of intensively and extensively managed habitats, allowing N availability to be studied in relation to soil and vegetation type, and also to variation in climate and in reactive N deposition from the atmosphere. Mineralisable N contents were correlated with deposition in extensively managed habitats but not in intensively managed habitats. The following statements apply only to extensively managed habitats. All habitats showed a similar increase in Nrm with N deposition. However, soil characteristics affected the relationship, and soil carbon content in particular was a major control on mineralisation. The Nrm stock increased more with N deposition in organic than in mineral soils. The nitrate proportion of Nrm also increased with N deposition but, conversely, this increase was greater in mineral than in organic soils. The measurements could be used as indicators of eutrophication, e.g. deposition rates of over 20 kg N ha− 1 y− 1 are associated with nitrate proportions of > 41% in a mineral soil (2% carbon), and with Nrm stocks of over 4.8 kg N ha− 1 in an organic soil (55% carbon). Both Nrm and nitrate proportion increased with mean annual temperature of the sampling location, despite consistent incubation temperature, suggesting that increasing temperatures are likely to increase the eutrophying effects of N pollution on semi-natural ecosystems. Diunduh dari: http://0-www.sciencedirect.com.precise.petronas.com.my/science/article/pii/S0048969711014914 Soil Moisture Control of Nitrogen Fixation Activity in Dry Tropical Casuarina Plantation Forest Alok K. Srivastava , R.S. Ambasht Journal of Environmental Management. Volume 42, Issue 1, September 1994, Pages 49–54 Nitrogenase activity nitrogen accretion to soil in two age groups of Casuarina equisetifolia plantation forest have been assessed. Our findings show that, in the summer months, the nitrogenase activity is at a minimum but, with the onset of rain even though it continues to be very warm, new nodulation starts and the peak of N2-fixing activity is soon reached. Multiple regression analysis showed soil moisture in the warm temperature condition as the major factor controlling the nitrogenase. Based on our finding, we are tempted to suggest that if adequate moisture through judicious irrigation management is made available in summer, the nodulation could be hastened and N2 fixation activity could be prolonged at a high level during 4 months of summer and 4 months of rains. Diunduh dari: http://www.sciencedirect.com/science/article/pii/S0301479784710590 . RELATION OF AVAILABLE SOIL NITROGEN TO RICE YIELD. Dolmat, M. T.; Patrick, W. H., Jr.; Peterson, F. J. Journal Soil Science 1980 Vol. 129 No. 4 pp. 229-237 The relationship between the available soil nitrogen and rough rice yields was then investigated. Total soil nitrogen varied widely, ranging from 540 to 5460 parts per million. The average percentage recovery of total soil nitrogen (representing the available soil nitrogen), as determined by the various methods used, also ranged widely, from as high as 16.74 percent, by the hot alkaline permanganate method, to the lowest value of 1.08 percent, by boiling soils in 0.01 M CaCl2 solution. Good correlation coefficients were obtained, especially among the anaerobic incubation and each of the other extraction methods used. The acid hydrolysis method correlated the least with the other extraction methods. Highly significant correlation coefficients were obtained between the rough rice yields from the untreated plots (plots that received no nitrogen) and the available soil nitrogen determined by all the extraction methods. The best correlation was obtained with the anaerobic incubation method (r = 0.622). The rough rice yield in plots receiving 56 or more kilograms of nitrogen per hectare did not exhibit a significant relationship with the available soil nitrogen. Relationships between the rough rice yields at zero and 28 kg/ha N and the available soil nitrogen, as determined by the anaerobic incubation method, were better described by curvilinear models than by linear ones. The relationship was also established between yield increase from application of 112 kg/ha N (the level of nitrogen generally considered near optimum for rice in Louisiana) and available soil nitrogen determined by the anaerobic incubation method. With some reasonable degree of accuracy, yield increase could be predicted from the graph signifying this relationship. Diunduh dari: http://www.cabdirect.org/abstracts/19801955633.html;jsessionid=587B71ACB1ACE2A89AB75354744EEFF9?gitCommit=4.13.19-9-g409ea67 . Nitrogen Dynamics and Indices to Predict Soil Nitrogen Supply in Humid Temperate Soils Mervin St. Luce , Joann K. Whalen , Noura Ziadi , Bernie J. Zebarth Advances in Agronomy. Volume 112, 2011, Pages 55–102 In humid temperate regions, soil N supply is dominated by in-season N mineralization because plantavailable N (NH4–N and NO3–N) is transformed to nonlabile forms or lost from the soil–plant system during fall and winter. The microbially mediated reactions that generate the soil N supply in agroecosystems are affected by system-specific conditions, including soil properties, agricultural management (crop rotation, tillage system, organic amendments), and most importantly, climate. Potentially mineralizable N (N0) determined from long-term soil incubation is regarded as the standard measure of soil N mineralization potential and may provide a good approximation of the soil N supply. However, this method is time consuming and not practical for routine use. Several chemical methods to estimate the N mineralization potential of soils are discussed in this chapter. The major limitation of chemical methods is that they cannot simulate the microbial-mediated release of plant-available N under field conditions. Consequently, any single chemical method may not be a good predictor of soil N supply. Thus, we suggest a holistic approach to estimate soil N supply in humid temperate regions, which involves (1) the use of a combination of N indices together with weather data and (2) identification and quantification of a specific fraction (s) of organic N that is the dominant contributor (s) to N supply in a particular system. Diunduh dari: http://www.sciencedirect.com/science/article/pii/B9780123855381000020 . Nitrogen Dynamics and Indices to Predict Soil Nitrogen Supply in Humid Temperate Soils Mervin St. Luce , Joann K. Whalen , Noura Ziadi , Bernie J. Zebarth Advances in Agronomy. Volume 112, 2011, Pages 55–102 Illustration of the nitrogen cycle in soil. Diunduh dari: http://www.sciencedirect.com/science/article/pii/B9780123855381000020 . Nitrogen transformations with special reference to gaseous N losses from zerotilled soils of Saskatchewan, Canada M.S. Aulakh , D.A. Rennie Soil and Tillage Research. Volume 7, Issues 1–2, May 1986, Pages 157–171 Cumulative gaseous N losses (N2 O + N2) measured with acetylene inhibition-soil core technique ranged from 1 to 7 kg ha−1 year−1 N for CT and from 12 to 16 kg ha−1 year−1 N for ZT fields. In both CT and ZT, gaseous N losse were 2–5 times higher for a wheat-fallow than a continuouswheat rotation. The denser surface soil and consistently higher moisture content of ZT fields were identified as the main reasons for higher rates of denitrification. The potential denitrification rates were markedly higher in ZT and the population of denitrifiers was up to six times higher than in the CT fields. Crop residues doubled the gaseous N losses. Temperature above 5°C did not alter denitrification rates nor did a wide range of mineral N. The contribution of lower soil horizons towards gaseous N losses was negligible. Mole fraction of N2O [N2O/(N2O + N2)] showed a reverse relationship with soil moisture and varied from 28 to 98% in the total gaseous N products. About 35% of autumn-applied 15N-labelled fertilizer N was lost via denitrification and 7–20% became immobilized by the following spring. Leaching was not responsible for the lower efficiency of fertilizer N. Due to adequate N fertilization and good straw mulch conditions, yield, N uptake and protein content of wheat were highest in the ZT system. The ZT system was also efficient in conserving more moisture from over-winter snowfall and rains. Diunduh dari: http://www.sciencedirect.com/science/article/pii/0167198786900152 . Effects of soil water content and nitrogen supply on the productivity of Miscanthus x giganteus Greef et Deu. in a Mediterranean environment Cosentino SL, Patane C, Sanzone E, Copani V, Foti S Industrial Crops and Products. [2007, 25(1):75-88] Miscanthus x giganteus is one of the most promising biomass crops for non-food utilisation. Taking into account its area of origin (Far East), its temperature and rainfall requirements are not well satisfied in Mediterranean climate. For this purpose, a research was carried out with the aim of studying the adaptation of the species to the Mediterranean environment, and at analysing its ecophysiological and productive response to different soil water and nitrogen conditions. A split plot experimental design with three levels of irrigation (I1, I2 and I3 at 25%, 50% and 100% of maximum evapotranspiration (ETm), respectively) and three levels of nitrogen fertilisation (0 kg ha-1: N0, 60 kg ha-1: N1 and 120 kg ha-1: N2 of nitrogen) were studied. The crop showed a high yield potential under well-watered conditions (up to 27 t ha-1 of dry matter). M. x giganteus, in Mediterranean environment showed a high yield potential even in very limited water availability conditions (more than 14 t ha-1 with a 25% ETm restoration). A responsiveness to nitrogen supply, with great yield increases when water was not limiting, was exhibited. Water use efficiency (WUE) achieved the highest values in limited soil water availability (between 4.51 and 4.83 g l-1), whilst in non-limiting water conditions it decreased down to 2.56 and 3.49 g l-1 (in the second and third year of experiment, respectively). Nitrogen use efficiency (NUE) decreased with the increase of water distributed (from 190.5 g g-1 of I0 to 173.2 g g-1 of I2); in relation to N fertilisation it did not change between the N fertilised treatments (N1 and N2), being much higher in the unfertilised control (177.1 g g-1). Radiation use efficiency (NUE) progressively declined with the reduction of the N fertiliser level (1.05, 0.96 and 0.86 g d.m. MJ-1, in 1994, and 0.92, 0.91 and 0.69 g d.m. MJ-1, in 1995, for N2, N1 and N0, respectively). Diunduh dari: http://europepmc.org/abstract/AGR/IND43888538