Chemical Names and Formulas Power point
advertisement
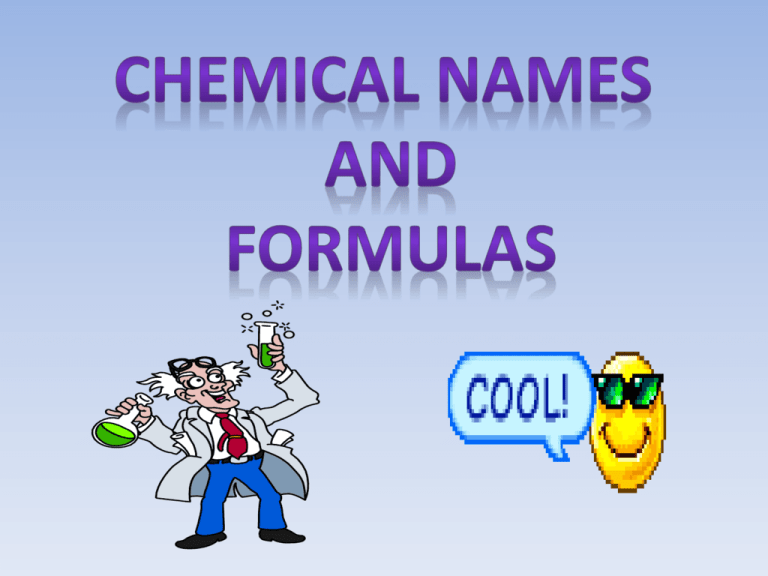
Significance of a Chemical Formula •Chemical formulas form the basis of the language of chemistry and reveal much information about the substances they represent. •To describe the atomic makeup of compounds chemists use systematic methods for naming compounds and for writing chemical formulas. •A chemical formula indicates the relative number of atoms of each kind in a chemical compound. •For a molecular compound, the chemical formula gives the number of atoms of each element contained in a single molecule of the compound. •Example: octane C8H18 The subscript after the C indicates that there are 8 carbon atoms in the molecule. The subscript after the H indicates that there are 18 hydrogen atoms in the molecule. •The chemical formula for an ionic compound represents one formula unit – the simplest ratio of the compound’s (+) ions and (-) ions. •Example: aluminum sulfate Al2(SO4)3 •Parentheses surround the polyatomic ion (SO4) to identify it as a unit. The subscript 3 refers to the Al2(SO4)3 unit. 2 aluminum atoms 3 sulfur atoms and 12 oxygen atoms •Remember ionic compounds exist as crystalline solids. •Aluminum sulfate crystal Monatomic Ions •Monatomic ions are ions formed from a single atom. •Monatomic cations are identified by the element’s name (Na+ is sodium). •When naming monatomic anions, you drop the ending of the element’s name and add –ide. •F-, Fluorine → Fluoride •Many of the transition metals lose different numbers of electrons and follow the Stock System of naming ions and elements. •Example: copper(I) → Cu+ or copper(II) → Cu2+ copper(I) ion – lost one electron copper(II) ion – lost two electrons Binary Ionic Compounds •Binary ionic compounds are compounds composed of two different elements. •The total number of positive and negative charges must be equal. •The cross over method is a method of balancing the charges between the ions in an ionic compound. •Use your Valence Graphic organizer and ion handout to help you with the charges on ions. Polyatomic Ions O O N O •In a polyatomic ion, two or more atoms are covalently bonded together. 1– O O N O •Together, they carry a charge. •When balancing charges in an ionic compound, the polyatomic ions acts as a single unit of charge. 1- 2+ Mg Mg(NO3)2 1- 1- 2+ Mg Mg(NO3)2 2+ 1- 1- 2+ 1- Mg the charges are balanced Mg(NO3)2 2+ 2- Remember a parentheses is placed around the polyatomic ion to identify it as a unit. Cross Over Method (or Criss-Cross Method) •The cross over method is a method of balancing the charges between the ions in an ionic compound. Example: aluminum oxide 1) Write the symbols for the ions. Al3+ O2- 2) Cross over the charges by using the absolute value of each ion’s charge as the subscript for the other ion. Al23+ O32- 3) Check the combined positive and negative charges to see if they are equal. 2 Al atoms x 3+ = +6 3 O atoms x 2- = -6 0 The correct formula is Al2O3. Naming Binary Ionic Compounds •The nomenclature, or naming system, or binary ionic compounds involves combining the names of the compound’s positive and negative ions. •The name of the cation is given first, followed by the name of the anion (-ide). •Example: Al2O3 aluminum oxide •Some elements such as iron, form two or more cations with different charges. •The Stock System of nomenclature uses a Roman numeral to indicate an ion’s charge. •The numeral is enclosed in parentheses and placed immediately after the metal’s name. •Example: Fe2+ - iron(II), Fe3+ - iron(III). Example: write the name for the following compound. SnI4 1) First determine the charge on the Sn ion. The iodine ion has a charge of 1- and the formula contains 4 I- ions therefore, the overall negative charge is 4-. 2) In order for the charges to equal the Sn ion must have a charge of 4+. 3) The name would be tin(IV) iodide. Compounds containing Polyatomic Ions •Most polyatomic ions are negatively charged and are oxyanions – contain oxygen. •Compounds containing polyatomic ions are named in the same way as binary ionic compounds. •Name the cation first, then the anion. •Example: Ca(OH)2 Name cation first, then the anion. calcium hydroxide •If more than one oxyanion is formed by the same two elements (Ex. N and O) the most common ion is given the ending – ate. •The one with one less oxygen ends in –ite. •Example: NO2- - nitrite, NO3-, nitrate. •Some elements can form more than two types of oxyanions. •Example: chlorine can form •ClO- (hypochlorite) •ClO2- (chlorite) •ClO3- (chlorate) •ClO4- (perchlorate) Naming Binary Molecular Compounds •The naming of molecular compounds is based on the use of prefixes (CO is carbon monoxide, CO2 is carbon dioxide, P4O10 is tetraphosphorus decoxide). •The less electronegative element is given first. It is given a prefix only if it contributes more than one atom to a molecule. •The second element is named by (a) prefix giving number of atoms contributed (b) the root of the name of the second element, and (c) the ending –ide. Numerical Prefixes Number 1 2 3 4 5 6 7 8 9 10 Prefix monoditritetrapentahexaheptaoctanonadeca- The prefix system is illustrated below. Prefix needed if first element contributes more than one atom + Name of first element Prefix indicating number of atoms contributed by second element. + Root name of second element + ide.