SCIENCE
advertisement

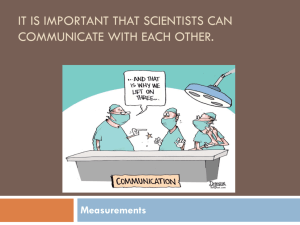
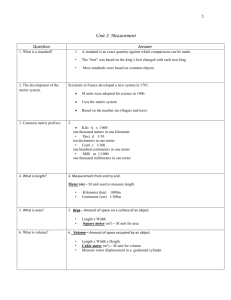
Science Classwork Metric Soup 9/13/2013 Instructions: create the nastiest, grossest, most horrendous soup possible. List your ingredient in the first column, the amount of each ingredient (liters/grams) in the second column, and then convert your amount to mL or mg in the third column. Remember that liquids are in liters and solids are in grams. Ingredient Amount mL/mg Metric Conversions (9/12/13) • Write the correct abbreviation for each metric unit. • 1) Kilogram _____ 4) Milliliter _____ 7) Kilometer _____ • 2) Meter _____ 5) Millimeter _____ 8) Centimeter _____ • 3) Gram _____ 6) Liter _____ 9) Milligram _____ • Try these conversions, using the ladder method. • 10) 2000 mg = _______ g 15) 5 L = _______ mL 20) 16 cm = _______ mm • 11) 104 km = _______ m 16) 198 g = _______ kg 21) 2500 m = _______ km • 12) 480 cm = _____ m 17) 75 mL = _____ L 22) 65 g = _____ mg • 13) 5.6 kg = _____ g 18) 50 cm = _____ m 23) 6.3 cm = _____ mm • 14) 8 mm = _____ cm 19) 5.6 m = _____ cm 24) 120 mg = _____ g 9/6/2013 Measurement Study Guide 1. What does S.I. stand for? 2. What is the S.I unit for … temperature ________ volume ________ length ________ mass _________ • 3. What does each prefix stand for? • Kilo ________ • Centi _______ • Milli _________ 4. What is mass? How do you measure mass? 5. What is volume? How do you measure liquid or solid volume? 6. What is density? How do you measure density? 9/5/2013 • Review Volume and Density • Measurement Lab 9/4/2013 • Review Mass • Measurement Lab 9/3/2012 • Newsletter • Virtual Mass Lab • Lab Record Sheet 8/30/2013 “3 -2 -1” • 3 things you know about the S.I. system • 2 things you have questions about • 1 important reason to understand the S.I. system Measure in Meters • Each group will get one meter stick. • Copy the objects to measure. • Work as a group to measure each object and record your measurements in centimeters or meters. 8/29/2013 • Anticipation guide – Write true or false A. Mass and weight are two words for the same measurement. B. Everyone in the world should understand the SI system of measurement because it is the standard system to measure across the world. C. If you find the mass and volume of an object, then you can find its density easily. D. The first country to adopt the metric system was France. Chunking the Text Read each section. Discuss with your group the main ideas of the section and write them in the space provided. Title of Section Tools SI Units Length Mass Volume Density Temperature Main Ideas from Section Tools • One way to collect data is to take measurements. To get the best measurements, you need the proper tools. Stopwatches, meter sticks, and balances are some of the tools you can use to make measurements. Thermometers can be sued to observe changes in temperature. • After you collect data, you need to analyze them. Calculators are handy tools to help you do calculations quickly. Or you might show your data in a graph or a figure. A computer that has the correct software can help you display your data. Of course, you can use a pencil and graph paper to graph your data. S.I. System • In the late 1700s, the French Academy of Sciences set out to make a simple and reliable measurement system. Over the next 200 years, the metric system was formed. This system is now the International System of Units (SI). Because all SI units are expressed in multiples of 10, changing from one unit to another is easy. Prefixes are used to express SI units that are larger or smaller than basic units such as meter and gram. For example, kilo- means 1,000 times, and milli- indicates 1/1000 times. The prefix used depends on the size of the object being measured. Length • To describe the length of an Olympicsized swimming pool, a scientist would use meters (m). A meter is the basic SI unit of length. Other SI units of length are larger or smaller than the meter by multiples of 10. For example, if you divide 1 m into 1000 parts, each part equals 1 millimeter (mm). *Length Activity* Mass • Mass is the amount of matter in an object. The kilogram (kg) is the basic SI unit for mass. The kilogram is used to describe the mass of large objects. One kilogram is 1,000 grams. So, the gram is used to describe the mass of small objects. Masses of very large objects are expressed in metric tons. A ton is 1,000 kg. *Mass Activity* • http://www.explorelearning.com/index.cfm? method=cResource.dspView&ResourceID=385 • http://www.explorelearning.com/index.cfm? method=cResource.dspView&ResourceID=653 Volume • Imagine that you need to move some lenses to a laser laboratory. How many lenses will fit into a crate? The answer depends on the volume of the crate and volume of each lens. Volume is the amount of space that something takes up. • Liquid volume is expressed in liters (L). Graduated cylinders are used to measure the volume of liquids. • Volumes of solid objects are usually expressed in cubic meters. To find the volume of a rectangular crate, multiply the length and the width and the height. Volume Activity • http://www.explorelearning.com/index.cfm? method=cResource.dspView&ResourceID=104 8 Density • Density is the amount of matter in a given volume. You cannot measure density directly. If you measure the mass and volume of an object, you have the information you need to find the density. Use the following equation. »D = m/V Temperature • The temperature of a substance is a measurement of how hot the substance is. Degrees Fahrenheit and degrees Celcius are used to describe temperature, but the SI unit for temperature is the Kelvin. Closing Activity • Look back at the anticipation guide. Answer the true false questions again on your paper beside your old answers. Circle your new answers. SCIENCE 8/21/2013 Graphic Organizer – Lab Safety • _________________ • _________________ • _________________ • _________________ •_________________ •_________________ •_________________ •_________________ • _________________ • _________________ • _________________ • _________________ General Glass Heat Chemicals •_________________ •_________________ •_________________ •_________________ G.O. Instructions • After I assign you to a group, your group will then follow these instructions: – 1. Think of all the lab safety rules that you can. Share them with your group. Discuss important ones to follow. Choose one person to write them on a piece of paper. – 2. Each person draw this G.O. on your own paper – 3. Put each rule into one of the categories – 4. You only need 2 rules in each category – 5. You will have 5 minutes to complete – 6. After 5 minutes, one person from the group will share with the class Jot Notes • Add rules under each category until you have 4 in each category as I go over the powerpoint.