Hess' Law PreLab
advertisement

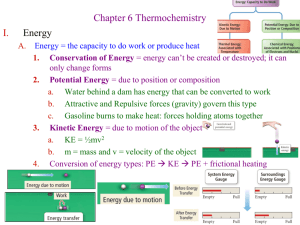
Hess’ Law PreLab I. Energy A. Energy = the capacity to do work or produce heat 1. Conservation of Energy = energy can’t be created or destroyed; it can only change forms 2. Potential Energy = due to position or composition a. Water behind a dam has energy that can be converted to work b. Attractive and Repulsive forces (gravity) govern this type c. Gasoline burns to make heat: forces holding atoms together 3. Kinetic Energy = due to motion of the object 4. Heat = q = energy transferred between objects at different temperatures a. Temperature = KE of particles in random motion b. Heat is not a substance, but our language often treats it that way B. Chemical Energy 1. Mechanical Energy = energy of the movement of objects 2. Chemical Energy = energy of the change in chemical bonds a. CH4(g) + 2O2(g) -------> CO2(g) + 2H2O(g) + heat b. Heat energy is liberated by rearranging the chemical bonds 3. Dividing up the Universe a. System = the specific reactants and products we are investigating b. Surroundings = everything else in the Universe 4. Heat flow in chemical reactions (heat = q = DH = Enthalpy) a. Exothermic = energy flows out of the system as heat (-DH) i. Products have a lower PE than the reactants ii. Heat can be viewed as a product iii. Heat released results in an increase in KE of surrounding particles b. Endothermic = energy flows into the system (+DH) i. N2(g) + O2(g) + heat -------> 2NO(g) ii. Heat can be viewed as a reactant iii. Heat absorbed results in less KE of the surrounding particles iv. Products have more PE than the reactants II. Hess’s Law A. Enthalpy is a State Function 1. Path doesn’t matter 2. As long as we know reactants and products, steps don’t matter for DH 3. Example: N2(g) + O2(g) -------> 2NO(g) DH1 = 180 kJ 2NO(g) + O2(g) -------> 2NO2(g) DH2 = -112 kJ N2(g) + 2O2(g) -------> 2NO2(g) DHtotal = 68 kJ 4. Hess’s Law a. You may sum steps in order to find overall DH b. The DH for the reverse reaction will simply change signs (+/-) c. If you multiply a reaction, you must multiply DH the same 5. Explanation a. Sign of DH depends on direction of heat flow. Heat flow is reversed if the overall reaction is reversed. Xe(g) + 2F2(g) -------> XeF4(s) + 251kJ (DH = -251kJ) XeF4(s) + 251kJ -------> Xe(g) + 2F2(g) (DH = +251kJ) b. DH is an extensive property = depends on the amount of substance (an intensive property = depends only on identity of the substance) Xe(g) + 2F2(g) -------> XeF4(s) + 251kJ (DH = -251kJ) 2Xe(g) + 4F2(g) -------> 2XeF4(s) + 502kJ (DH = -502kJ) B. Examples 1. Calculate DH for the conversion of graphite to diamond using the known DH’s for the combustion of graphite and diamond. a. Cg(s) + O2(g) -------> CO2(g) DH = -394kJ Cd(s) + O2(g) -------> CO2(g) DH = -396 kJ b. Cg(s) + O2(g) -------> CO2(g) CO2(g) -------> Cd(s) + O2(g) Cg(s) -------> Cd(s) DH = -394kJ (reverse) DH = +396 kJ DH = +2 kJ 2. Today’s Experiment: Find DH for oxidation of Mg a. Reactions 1. Mg(s) + 2HCl(aq) -------> Mg2+(aq) + H2(g) + 2Cl-(aq) 2. Mg2+(aq) + H2O(l) + 2Cl-(aq) -------> MgO(s) + 2HCl(aq) 3. H2(g) + 1/2O2(g) -------> H2O(l) Total: Mg(s) + 1/2O2(g) -------> MgO(s) b. Accepted value for this reaction is DH = -602 kJ/mol DH1 = exp DH2 = exp DH3 = exp DHtotal = ? 3. Procedure a. Run reaction #1 in our calorimeter Do twice b. Calculate DH1 = (4.18 J/goC)(mtotal)(DT) = ____J c. Convert to kJ/mol: (DH1 x 1kJ/1000J)/(mol of Mg) d. Do the same thing for the reverse of Reaction #2 Do twice e. Remember to change the sign of the DH2 that you get f. The DH3 is found in your text Appendix = -286 kJ/mol g. Calculate DHtotal = DH1 + DH2 + DH3 Avg Dev = 4. Deviation from the mean = |DHi – DHavg| (Dev1 + Dev2)/2 Incident: Explosion of Nitric Acid Waste Incident: Explosion of Liquid Nitrogen Tank



![[SO2]2[O2] [SO3]2 524.4K• 462.9K• 8.314 J mol•K • ln125.4 61.5K](http://s3.studylib.net/store/data/008432217_1-f6f0ddc631a0ec89f84a5e786b3339ef-300x300.png)






