Slides - American Heart Association
advertisement

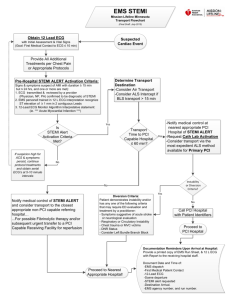
ACTION Registry-GWTG New User Training Webinar February 7,2013 Purpose of ACTION Registry-GWTG • National surveillance system for high-risk AMI patients admitted with STEMI/NSTEMI: – Assess characteristics, treatments, and outcomes of this patient population – Optimize outcomes and management of AMI patients through implementation of ACC/AHA evidence-based guideline recommendations in clinical practice – Facilitate efforts to improve quality and safety of ACS patient care; and investigate QI methods The History: ACTION RegistryGWTG • ACTION Registry transitioned from CRUSADE and NRMI Registries • January 2007 ACTION was established • May 2008 ACTION merged with AHA GWTG CAD to become ACTION Registry-GWTG • Current membership of 800 Hospitals • 500,000 records submitted Data Collection Options Web-Based Data Capture – Secure, password-protected data entry system – Free NCDR data collection tool – Interoperability from Cath/PCI Registry to ACTION • Vendor-Based Data Capture – Data submitted via encrypted, password-protected file – Interoperability between ACTION and Cath/PCI Registry www.ncdr.com Participant Log In The ACTION Registry-GWTG Webpage Call the American Hospital Association 1-800-424-4301 The Dashboard • eReports • Comparator Dashboard eReports Comparator • Standard Reports • Executive Summary Metrics • “Drill Downs” • Define peer groups • Facility attribute filters Technical Data Dictionary Outcomes Report Companion Guide Inclusion Population • Acute Myocardial Infarctions-STEMI & NSTEMI • Patient must present to 1st Facility with symptoms of ACS, within 24 hours of arrival • Patient must have positive ECG- ST elevation, new LBBB, or documented Posterior MI OR • Positive Biomarkers- Troponin or CK-MB within 24 hours of arrival • Transfer In patients- STEMI must arrive within 72 hours, NSTEMI within 24 hours • If presents with any other symptoms, or procedures, the patient is excluded Choosing the Correct Form Premier Form or Limited Form Every Hospital Has The Option To Use Either Form ACTION Registry-GWTG Premier Form • Complete quarterly Outcome Report for benchmarking – Report on 17 Core Performance Measures – Report on 12 Quality Metrics • Sites are Eligible for Higher Level of Recognition Program ACTION Registry-GWTG Limited Form • 50% of full ACTION data set • Limited quarterly Outcome Report for benchmarking – Report on 17 Core Performance Measures – Report on 7 Quality Metrics • Lower level of Recognition Limited Form: Pros and Cons Pros Cons Fewer Data Elements No Excessive dosing Reports for Anticoagulants Less time required for data abstraction and entry Lower Level of Recognition Accommodating for Non PCI Centers Limited Quarterly Outcomes Report Not all the metrics are included Great form for new sites to start Premier Form: Pros and Cons Pros Detailed Quarterly Excessive Dosing Reports for Anticoagulants Higher level of Recognition Robust Data Set Full Quarterly Outcomes Report Cons More time required for data abstraction and entry Answering fields that are less likely to pertain to Non-PCI Centers Demographics Cardiac Status & History Medications Anticoagulants Procedures Reperfusion Strategy Clinical Events & Biomarkers Labs Discharge Section K- Optional Elements Data Quality Reports (DQR) Data Assessment Results Failed Completeness Assessment ACTION Registry-GWTG National Data Slide Sets Produced every 6 months Use of Reperfusion Therapy for STEMI STEMI N = 21,978 Reperfusion Not Eligible for Reperfusion Therapy N = 17,711 (81%) No Reperfusion – Contraindication Listed N= 2,866 (13%) No Contraindication Listed N = 1272 (6%) Primary PCI – 86%* Fibrinolytics – 13%* Both PCI + Lytics – 1%* 93% of eligible patients reperfused ACTION Registry-GWTG DATA: July 1, 2008 – June 30, 2009 * Among patients receiving reperfusion Time (min) ACTION Door-to-Balloon Times – Median Times for Transfer In and Non-Transfer In Patients 250 240 230 220 210 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0 236 169 123 223 215 212 158 151 156 116 113 120 103 79 62 Q1 07 102 78 60 Q2 07 Transfer in DTB Times 96 95 75 57 74 Q3 07 57 Q4 07 Non-Transfer in DTB Times STEMI Door-to-Balloon Times – Time (min) Median Times for Transfer In and Non-Transfer In Patients 250 240 230 220 210 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0 182 165 130 102 123 88 97 84 157 150 120 117 96 82 94 79 70 67 66 64 53 52 51 50 Q1 08 Q2 08 Transfer in DTB Times Q3 08 Q4 08 Non-Transfer in DTB Times STEMI – Door to Balloon and Door to Needle Times: Cumulative 12 Month Data 100% 87% 80% 67% 60% 40% 20% 20% 0% DTB <= 90 min Non-Transfer In DTB <= 90 min Transfer In DTN <= 30 min - All ACTION Registry-GWTG DATA: July 1,2008 – June 30, 2009 DTB = 1st Door to Balloon for Primary PCI DTN = Door to Needle for Lytics NSTEMI Acute Medication Overdosing Trends 30% 25% 20% UFH* 15% LMWH# GP Iib-IIIa 10% 5% 0% * Infusion (> 15 units/kg/hr) or bolus (> 70 units/kg) # Initial dose (> 1.05 mg/kg) or total 24 hr dose (> 10 mg over recommended) Q3 2008 Q4 2008 Q1 2009 Q2 2009 ACTION Registry-GWTG DATA: July 1, 2008 – June 30, 2009 Quarterly Outcomes Reports Composite Measure Composite Measure Overall AMI Performance Graph Performance Measures Acute/In-hospital Measures Aspirin Arrival STEMI - Any reperfusion (PCI or Lytic) STEMI - Lytic -Door to Needle (Median Time and % <30min) STEMI - PCI – D2B (Median Time and % <90min STEMI - D2B Transfer in (Median Time) LVSD Evaluation Discharge Measures Aspirin B-blocker ACE or ARB (EF <40%) Statin for LDL ≥100mg/dL Smoking cessation (among smokers) Cardiac rehabilitation Performance Measure Graph: Aspirin at Arrival Quality Metrics ACTION Metrics Door to EKG (within 10 min) STEMI- Acute ADP Receptor Inhibitor Therapy within 24 hours of arrival_ Revascularized Patients Discharged on ADP Receptor Inhibitors ADP Receptor Inhibitors Prescribed at Discharge for Medically Treated Patients LDL assessment (in-hospital) NSTEMI - Excessive Initial UFH Dosing (>70 U/kg bolus, >15 U/kg/min infusion Excessive Initial Enoxaparin Dosing (SQ >1.05 mg/kg) Excessive Initial GP IIb/IIIa Dosing (Full doseTirofiban if CrCl<30& Full dose Eptifibatide CrCl <50, or dialysis with either) STEMI - Anticoagulant- UFH, enoxaparin, bivalarudin or fondaparinux (first 24 hours) Aldosterone Blocking Agents at Discharge(EF<40%, with DM, or HF) ACTION Registry-GWTG Recognition Program Recognition Criteria • Patient Volume – 10 NSTEMI within each quarter; and/or – 10 STEMI within past quarter • Must maintain uninterrupted data submission for Q1 – Q4 • 90% compliance Recognition Thresholds Must meet compliance on composite measures Participate in Platinum 90% compliance >= 8 consecutive quarters entering data Premier Gold 90% compliance >= 8 consecutive quarters entering data Premier or Limited Silver 90% compliance >= 4 consecutive quarters entering data Premier or Limited Award Levels Criteria for STEMI’s • STEMI composite: – – – – – – – ASA on Arrival DTN<=30 minutes DTB<=90 minutes) discharge ASA discharge beta-blocker, discharge ACE-I/ ARB (ideal patients) discharge statin (exclude if contraindicated or LDL<100mg/dl and not discharged on statin) – smoking cessation counseling, – cardiac rehabilitation, Criteria for NSTEMI’s • NSTEMI composite: – – – – – ASA on Arrival discharge ASA discharge beta-blocker discharge ACE-I/ ARB (ideal patients) discharge statin (exclude if contraindicated or LDL<100mg/dl and not discharged on statin) – smoking cessation counseling – cardiac rehabilitation (800) 257-4737 or email ncdr@acc.org Thank you for your participation in ACTION Registry-GWTG!