lec.4-426 (1)
advertisement

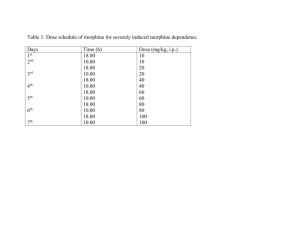
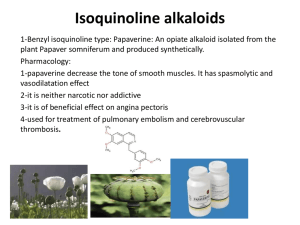
MEDICINAL CHEMISTRYIII Lecture 4 Sat. 19/ 5/ 1432H Analgesics The analgesics may be divided into two main classes: a) Opium and its alkaloids [morphine & related compounds] ……..Narcotics Analgesics or Opiate Drugs b) Non-Opiate Drugs [Aspirin & Phenacetin] ……… ……..Analgesic Antipyretics Centrally Acting Analgesic (Narcotic analgesic) The term centrally acting analgesic is used for compounds which inhibit the pain reaction within the central nervous system. Opium contains more than 25 alkaloids. There are two types of ring systems found in opium alkaloids Phenatheren ring system e.g. Morphine, Codeine, Thebaine Isoquinoline ring system e.g. Papaverine Centrally Acting Analgesic Opioid agonists a. Morphine and morphinan derivatives e.g. Morphine, Codeine ………… I. b. Piperidine derivatives Mepiridine and congeners Methadone and congeners Other structures e.g. Tramadol II Mixed Opioid Agonist-Antagonist and Partial Agonists e.g. Nalorphine II Opioid Antagonists e.g. Levallorphan III Nonopioid compounds e.g. Nefopam Opioid Agonists Morphine 7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol It is the principle alkaloid obtained from the dried latex (opium) from the unripe fruit of poppy (papaver somniferum) Morphine is one of the most effective pain killers in medicine. It is used in treating dull constant pain. Other effects: Respiratory depression Constipation Tolerance and physical dependence Euphoria Nausea and vomiting Dull Pupil constriction Biliary colic Flushing and warming 1, 3, 5, are the most dangerous side effects. Withdrawal symptoms are also dangerous, they include anorexia, pupil dilatation, chills, excessive sweating, cramps, muscle spasms, irritability, tremors etc…………….. Structure activity relationships: Stereochemistry: Natural morphine is levo (-), the dextro isomeris has been synthesized and it is devoid of analgesic and other opioid activities. 1. Alkylation of OH at C-3 Methylation Codeine Codeine has less analgesic activity & used mainly as antitussive drug. 7,8-didehydro-4,5-epoxy-3-methoxy-17-methylmorphinan-6-ol Codeine …………… Prodrug…………..metabolized ……………Morphine Methylation (CH3I) Codiene Morphine O-demethylation (HI) Ethylation Dionine (ethyl morphine) 7,8-didehydro-4,5-epoxy-3-ethoxy-17methylmorphinan-6-ol Dionine is used in ophthalmology as analgesic, especially in glucoma Alkylation with morphoine ethyl chloride Pholcodine CH3 N O O O N 7,8-didehydro-4,5-epoxy-3-O- (2-morphoinoethyl)-17methylmorphinan-6-ol Pholcodine used as antitussive OH 2. Acetylation: at C-3 & C-6 (Diamorphine, Heroin) (diacetyl morphine) Characters: more potent analgesic than morphine due to its high lipid solubility. - more addictive. - weaker antitussive. - metabolism ………….. 6-acetyl morphine which is more active than morphine. …………… diacetylation of acetyl group at C-3 & C-6 giving morphine. - 7,8-didehydro-4,5-epoxy-3,6-diacetyloxy-17-methylmorphinan. 7,8-double bond Reduction of 7,8 double bond ……………….dihydromorphine ………………..dihydrocodiene 4,5-epoxy-17-methylmorphinan 3,6-diol is more active than morphine 4,5-epoxy-3-methoxy-17-methylmorphinan-6-ol antitussive, analgesic If the reduction is accompanied by oxidation of OH at C-6 to ketone…………………….. activity toxicity Hydromorphone 4,5-epoxy-3-hydroxy-17methylmorphinan-6-one Hydrocodone 4,5-epoxy-3-methoxy-17methylmorphinan-6-one