plasma - Mr. Cramer
advertisement

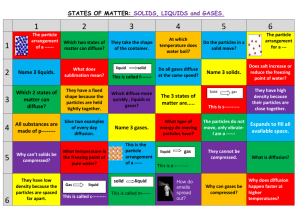
CHEMISTRY THE FOUR STATES OF MATTER STATES OF MATTER THE FOUR STATES OF MATTER FOUR STATES SOLID LIQUID GAS PLASMA THE FOUR STATES OF MATTER BASIS OF CLASSIFICATION OF THE FOUR TYPES BASED UPON PARTICLE ARRANGEMENT BASED UPON ENERGY OF PARTICLES BASED UPON DISTANCE BETWEEN PARTICLES DIVIDE YOUR PAPER INTO 4 SECTIONS • Label them Solid, Liquid, Gas, Plasma • You are to record different observations you have of each state. • You know that all matter is made of small particles. Make a hypothesis about what the particles look like with in matter. SOLIDS PARTICLES OF SOLIDS ARE TIGHTLY PACKED PARTICLES VIBRATE ABOUT A FIXED POSITION. SOLIDS HAVE A DEFINITE SHAPE AND A DEFINITE VOLUME. ON YOUR PAPER… • Draw what you think the arrangement of particles in a solid look like. • Fill in the chart with the properties (arrangment, shape, volume, movement) of a solid SOLIDS PARTICLE MOVEMENT EXAMPLES STATES OF MATTER LIQUIDS PARTICLES OF LIQUIDS ARE TIGHTLY PACKED ARE FAR ENOUGH APART TO SLIDE OVER ONE ANOTHER. LIQUIDS HAVE A VARIABLE SHAPE AND A DEFINITE VOLUME. ON YOUR PAPER… • Draw what you think the arrangement of particles in a liquid look like. • Fill in the chart with the properties (arrangement, shape, volume, movement) of a liquid Chumbler - Properties of Matter 9 STATES OF MATTER LIQUIDS PARTICLE MOVEMENT EXAMPLES Chumbler - Properties of Matter STATES OF MATTER GASES PARTICLES OF GASES ARE VERY FAR APART RANDOMLY PARTICLES MOVE FREELY. GASES HAVE A VARIABLE SHAPE AND A VARIABLE VOLUME. ON YOUR PAPER… • Draw what you think the arrangement of particles in a gas look like. • Fill in the chart with the properties (arrangement, shape, volume, movement) of a gas Chumbler - Properties of Matter 12 States of Matter GASES PARTICLE MOVEMENT EXAMPLES 13 STATES OF MATTER PLASMA PARTICLES OF PLASMA ARE VERY FAR APART AND MOVE RANDOMLY A PLASMA IS AN IONIZED GAS. A PLASMA IS A VERY GOOD CONDUCTOR OF ELECTRICITY AND IS AFFECTED BY MAGNETIC FIELDS. PLASMA, LIKE GASES HAVE A VARIABLE SHAPE AND A VARIABLE VOLUME. 14 ON YOUR PAPER… • Draw what you think the arrangement of particles in a plasma look like. • Fill in the chart with the properties (shape, volume, movement) of a plasma Chumbler - Properties of Matter 15 STATES OF MATTER PLASMA PARTICLES THE NEGATIVELY CHARGED ELECTRONS (YELLOW) ARE FREELY STREAMING THROUGH THE POSITIVELY CHARGED IONS (BLUE). PLASMA EXAMPLES 17 • What does it mean for something to be compressed? Which of the 4 states of matter do you think can be compressed and why? Chumbler - Properties of Matter 18 MOLECULAR EXPLANATION FOR PROPERTIES OF SOLIDS SOLIDS HAVE A DEFINITE SHAPE AND A DEFINITE VOLUME BECAUSE THE MOLECULES ARE LOCKED INTO PLACE. SOLIDS ARE NOT EASILY COMPRESSIBLE BECAUSE THERE IS LITTLE FREE SPACE BETWEEN MOLECULES. SOLIDS DO NOT FLOW EASILY BECAUSE THE MOLECULES CANNOT MOVE/SLIDE PAST ONE ANOTHER. 19 MOLECULAR EXPLANATION FOR PROPERTIES OF LIQUIDS LIQUIDS HAVE A VARIABLE SHAPE BECAUSE THE MOLECULES CAN SLIDE PAST ONE ANOTHER. LIQUIDS ARE NOT EASILY COMPRESSIBLE AND HAVE A DEFINITE VOLUME BECAUSE THERE IS LITTLE FREE SPACE BETWEEN MOLECULES. LIQUIDS FLOW EASILY BECAUSE THE MOLECULES CAN MOVE/SLIDE PAST ONE ANOTHER. 20 States of Matter MOLECULAR EXPLANATION FOR PROPERTIES OF GASES GASES HAVE A VARIABLE SHAPE AND A VARIABLE VOLUME BECAUSE THE MOLECULES CAN MOVE PAST ONE ANOTHER. GASES ARE EASILY COMPRESSIBLE BECAUSE THERE IS A GREAT DEAL OF FREE SPACE BETWEEN MOLECULES. GASES FLOW VERY EASILY BECAUSE THE MOLECULES RANDOMLY MOVE PAST ONE ANOTHER. 21 STATES OF MATTER MOLECULAR EXPLANATION FOR PROPERTIES OF PLASMAS PLASMAS HAVE A VARIABLE SHAPE AND A VARIABLE VOLUME BECAUSE THE PARTICLES CAN MOVE PAST ONE ANOTHER. PLASMAS ARE EASILY COMPRESSIBLE BECAUSE THERE IS A GREAT DEAL OF FREE SPACE BETWEEN PARTICLES. PLASMAS ARE GOOD CONDUCTORS OF ELECTRICITY AND ARE AFFECTED BY MAGNETIC FIELDS BECAUSE THEY ARE COMPOSED OF IONS (NEGATIVELY CHARGED ELECTRONS AND POSITIVELY CHARGED NUCLEI). THE FOUR STATES OF MATTER THE CLASSIFICATION AND PROPERTIES OF MATTER DEPEND UPON MICROSCOPIC STRUCTURE PARTICLE ARRANGEMENT PARTICLE ENERGY PARTICLE TO PARTICLE DISTANCE 23