Naming Compounds Notes
advertisement

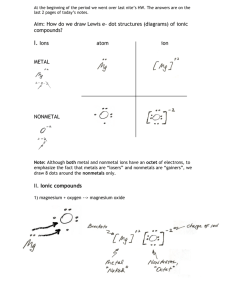
Naming Compounds Chemical Bonding: The force that holds two atoms together is called a chemical bond. This bond may be formed many different ways but for now we will only discuss two types of bonds, ionic and covalent. An element’s properties and behavior depends on their valence electrons. These outer electrons are also responsible for how atoms bond to another. Formation of Positive Ions: Groups 1, 2, and 13 will lose electrons to achieve stable octets. This process is favourable because it requires far less energy than gaining multiple electrons. Formation of Negative Ions: Group 15, 16, and 17 have greater attraction to electrons and thus try to gain more electrons to get a stable octet. Polyatomic Ions: A group of atoms that have a charge. For example, OH- (hydroxide). Naming with Only One Type of Atom: To name anything with only one type of atom just use the name that appears on the periodic table of elements. However, there are some exceptions to this rule. 1. There are 7 diatomic elements which include H2, N2, O2, F2, Cl2, Br2, and I2. These elements are found in nature bonded to each other. These still use the names given in the periodic table. 2. Monatomic cations use the element name followed by the word ion. Ex. Cs+ is named cesium ion. 3. Monatomic Anions take their name from the root of the element plus the “ide” suffix. All anions can also be found on your reference sheet. Ex. Br- is named bromide ion. 4. Phosphorous is found in nature in a group of 4 (P4) but is still named phosphorous. Practice- Name the following Atoms: Li Br2 Tc Ca 2+ P4 Rn S2Titanium Sodium ion Phosphide ion Naming with One Type of Atom Practice: a)Cr b) Sn c) Bismuth d) Mercury e) S2f) Cs+ g) B3+ h) Mg2+ i) Ij) Hk) chloride ion l) lithium ion m) Oxide ion n) hydrogen ion o) Nitride ion Naming Ionic Compounds Ionic Bonds: These bonds are formed when ions are joined due to electrostatic attraction. Typically a metal and a non-metal form ionic bonds. For example, sodium transfers an electron to chlorine gas and forms sodium chloride. If an ionic bond occurs between a metal and oxygen it is an oxide (Fe2O3). Most other ionic bonds are salts. The number of electrons lost must equal the number of electrons gained and the overall charge must equal zero. So if calcium bonds with fluorine, there must be 2 fluorine atoms to take 1 electron each. Likewise if sulfur bonds with sodium, there must be two sodium atoms to give each sulfur atom 1 electron. Naming Binary Ionic Compounds (Non-metal and metal): FORMULA 1. Write the symbol of the metal or positive ion first. 2. Write the symbol of the negative ion. Common negative ions and nonmentals are listed on your reference sheet. 3. Overall charge must equal 0. Aluminum chloride is written as AlCl3 not AlCl. Al3+ Mn4+ Co3+ Cu2+ Pb4+ ClO2IN3FORMULA TO NAME 1. Write the name of the positive ion followed by the name of the negative ion. Ex. MgO is named magnesium oxide, Na2S is named sodium sulfide. NaOH is named sodium hydroxide 2. Common negative ions and nonmentals are listed on your reference sheet. Take these names as they are! DO NOT change them. Ex. Fluorine becomes fluoride and bromine becomes bromide. 3. Many transition metals on the periodic table have more than one possible charge, and therefore when we look at an ionic compound that contains a multivalent metal, we need to be able to identify the charge of the metal ion. If the metal forms more than one type of cation, the charge has to be indicated using roman numerals. Roman Numerals One (I) Five (V) Two (II) Six (VI) Three (III) Seven (VII) Four (IV) Eight (VIII) 2+ Ex. Fe becomes Fe (II) and Fe3+ becomes Fe (III). If we have a formula of FeCl2 which of the following would be the correct name? iron (II) chloride OR iron (III) chloride Practice – Name the following ionic compounds: NaCl: sodium chloride (also known as table salt) AlBr3: aluminum bromide NiO: nickel (II) oxide PdI4: palladium (IV) iodide PbCl2: lead (IV) chloride Name to Formula 1. Write the name of the cation. Use the roman numerals to predict the charge of each atom. Remember, the Roman numeral is used to help identify the charge of the atoms. 2. Write the name of the anion as shown on your reference sheet. Practice – Write the formula of the following ionic compounds: Sodium oxide: Na2O Potassium chloride: KCl Scandium nitride: ScN Cobalt (III) sulfide: Co2S3 Naming Ionic Bonds with a Polyatomic Ion: Formula to Name: 1. If the polyatomic compound is the cation then use the name found on your reference sheet Ex. Ammonium (NH4+) 2. If the polyatomic compound is the anion find the name on your reference sheet. Use that name and do not change the ending. When using an ion there must be brackets around the entire ion if there is more than one of that ion. Ex. Magnesium cyanate is written as Mg(OCN)2 NOT as MgOCN2 Ex. ClO3- (chlorate) 3. Again, the charges must equal 0. If they do not you must balance them. Remember, the polyatomic ion is a compound hooked together which means you must use brackets around the term to balance it. Ex. Magnesium nitrate is written as Mg(NO3)2 Al3+ H30+ NH4+ Co3+ Cu2+ Pb4+ SCNSO42PO42S2O32- Al(SCN)3 Al2(SO4)3 Al2(PO4)3 Al2(S2O3)3 H3OSCN (H3O)2SO4 (H3O)2PO4 (H3O)2S2O3 NH4SCN (NH4)2SCN (NH4)2PO4 (NH4)2S2O3 Co(SCN)3 Co2(SO4)3 Co2(PO4)3 Co2(S2O3)3 Cu(SCN)2 CuSO4 CuPO4 CuS2O3 Pb(SCN)4 Pb(SO4)2 Pb(PO4)2 Pb(S2O3)2 Practice – Name the following ionic bonds with polyatomic ions: NaC2H3O2: sodium acetate H2O2: hydrogen peroxide K2HPO4: potassium hydrogen phosphate Pt(CrO4)2: platinum (IV) chromate Ca(BrO2)2: magnesium bromite NaOH: sodium hydroxide H3OBr: hydronium bromide Name to Formula: 1. Write the name of the cation. Use the roman numerals to predict the charge of each atom. Remember, the Roman numeral is used to help identify the charge of the atoms. 2. Write the name of the anion as shown on your reference sheet. Practice – Write the formula of the following ionic compounds: Sodium dihydrogen phosphate: Na2H2PO4 Cesium permanganate: CsMnO4 Ammonium sulfide: (NH4)2S Zinc thiocynate: Zn(SCN)2 Naming Covalent Molecules Covalent Bonds: The second type of bond is a covalent bond. These bonds occur when both atoms need electrons to get to a stable octet. Instead of transferring electrons they share them. These usually occur with a non-metal and a non-metal. For example, F has an electron configuration of 1s22s22p5 which means it has 7 valence electrons and must gain one more to achieve a full octet. It achieves this by sharing with another fluorine atom to form F2. It is important to note that some compounds require more than one covalent bond to achieve an octet, like N2 or O2. Because these compounds share electrons NO CHARGES are associated with each atom in the compound. Naming Covalent Compounds (Non-metal and Non-metal): Formula to Name: 1. The element farthest to the left of the periodic table is named first. If they are in the same column then the one with the most protons is named first. 2. Add the appropriate prefix to the front name (unless the prefix is mono-). This is used to indicate the number of atoms in each compound. Mono- is not used if there is only one atom of the first element. Ex. CO2 is named carbon dioxide NOT monocarbon dioxide (mono is not used on the first element) B3Br is name triboron monobromide (mono is used on the second one) **Prefixes found on reference sheet. 3. The second uses a prefix and add an “ide” to the ending of the name. Ex. Tetrachloride Practice – Name the following covalent compounds: CO3: carbon trioxide SF6: sulfur hexafluoride CO: carbon monoxide As2O3: diarsenic trioxide IF5: iodine pentafluoride N2O4: dinitrogen tetraoxide Name to Formula: Use the rules above in order to predict the formula: Dichlorine monoxide: Cl2O Silicon dioxide: SiO2 Dicarbon trioxide: C2O3 Carbon monofluoride: CF Pentanitrogen trioxide: N5O3 Heptacarbon trinitride: C7N3 Octaoxygen dinitride: O8N2 Naming Hydrates Formulas with a ●H2O These are compounds that have water molecules bonded to them to create a more stable structure. The stable structure may be required to ship the compound from place to place safely. 1. Use the previous rules to name the compound. 2. Attach ●H2O (named hydrate) to the end of the compound with the appropriate prefix to indicate the number of water molecules. Ex. NaNO3●3H2O is named sodium nitrate trihydrate Pb(NO3)2 ●5H2O is named lead(II) nitrate pentahydrate Practice – Name the following compounds containing hydrates: CaCl2●6H2O: calcium chloride hexahydrate Co(NO3)2●6H2O: cobalt (II) nitrate hexahydrate Cu(NO3)2●3H2O: copper (II) nitrate trihydrate Practice – Use the same rules to predict the formula: Ammonium sulfide heptahydrate: (NH4)2S●7H2O Iron (II) silicate pentahydrate: FeSiO3 ● 5H2O Lead (IV) nitrate octahydrate: Pb(NO3)4 ● 8H2O Copper (II) chloride trihydrate: CuCl2 ● 3H2O Gold (III) hydride pentahydrate: AuH3 ● 5 H2O Naming Acids Naming Binary Acids (Hydrogen and 1 Other Element): To name the compound first determine the ending of the root element: Rule #1 –If the anion’s name ends with “-ide”: o Add the prefix “hydro –.” o Replace the “-ide” ending with “-ic”. o Follow the name with the word acid. Rule #2 –If the anion’s name ends with “-ite”: o Replace the “-ite” ending with “-ous”. o Follow the name with the word acid. Rule #3 –If the anion’s name ends with “-ate”: o Replace the “-ate” ending with “-ic”. o Follow the name with the word acid. Ex. HBr is named hydrobromic acid HIO3 is named iodic acid HCrO3 is named chromous acid HCl is named hydrochloric acid HCN is name hydrocyanic acid Practice – Name the following binary acids: HIO3: iodic acid HF: hydrofluoric acid H3PO3: phosphorous acid H2CO3: carbonic acid H2Cr2O7: dichromic acid H2Se: hydorselenic acid Give the formula of the following acids: Nitrous acid: HNO2 Hyrdoselenic acid: H2Se hydrosulfuric acid: H2S Chromic acid: H2CrO4 Selenous acid: H2SiO3