Lewis Dot Structures of Ionic Compounds
advertisement

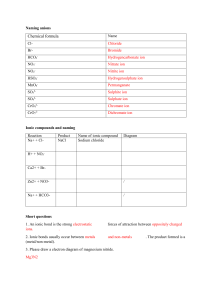
At the beginning of the period we went over last nite’s HW. The answers are on the last 2 pages of today’s notes. Aim: How do we draw Lewis e- dot structures (diagrams) of ionic compounds? I. Ions atom ion METAL NONMETAL Note: Although both metal and nonmetal ions have an octet of electrons, to emphasize the fact that metals are “losers” and nonmetals are “gainers”, we draw 8 dots around the nonmetals only. II. Ionic compounds 1) magnesium + oxygen --> magnesium oxide 2) potassium + sulfur --> potassium sulfide Not remember: "like charges repel, opposite charges attract" 3) aluminum + chlorine --> aluminum chloride It doesn't matter if the chloride ion in the middle is above or below the aluminum ion. See next page for review of last night's HW. SEE POINTS MADE IN LESSON IN REVIEW OF THIS HW BELOW