Equation Writing In Class Practice Pt. 2
advertisement

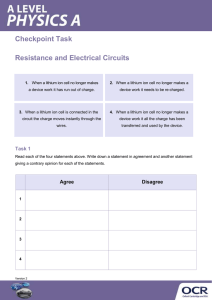
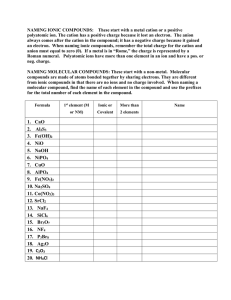
AP Chemistry In Class Equation Writing – Part 2 Directions: For each of the following reactions 1-8 write a balanced net ionic equation and answer the follow-up question. 1. Solid calcium oxide is heated in the presence of sulfur trioxide gas. If phenolphthalein is dropped into an aqueous solution of calcium oxide, what color will the resulting solution be? 2. 2 - Butanol is completely burned in air. If incomplete combustion of 2-butanol occurred, how many different carbon containing compounds would form? What are they? 3. A suspension (solid) of copper(II) hydroxide is treated with an excess of ammonia gas. What is the hybridization of the nitrogen atom in ammonia? 4. Solid potassium chlorate is strongly heated. Which acid is stronger, chloric or perchloric? Explain why. 5. A solution of ammonium sulfate is added to a saturated solution of barium hydroxide. Briefly describe the most effective way of making a saturated barium hydroxide solution. 6. Lead foil is immersed in silver nitrate solution. Why is silver predominantly found as a +1 charge in nature? 7. A strip of magnesium metal is heated strongly in pure nitrogen gas. Would the magnesium or sodium ion contribute more to Lattice Energy? Explain. 8. Solid lithium hydride is added to water. What is the oxidation number of the hydrogen in lithium hydride? 9. Circle (on this sheet) the ionic compounds below that are insoluble in water AgCl Ba(OH)2 NH4NO3 FeS Ca3(PO4)2 Fill in the blank on this sheet below. 10. a. When the MnO4- ion reduces in acid, the most common manganese ion formed is _______ b. When the Cl- ion oxidizes, the most common chloride compound formed is _______ c. If hydrogen peroxide reduces, what is the most common compound formed?________ 11. What is the general formula for determining the formula of an alkane?__________an alkyne?___________ 12. If a metallic carbonate decomposes, what are names of the products formed? _______________________