course outline
advertisement
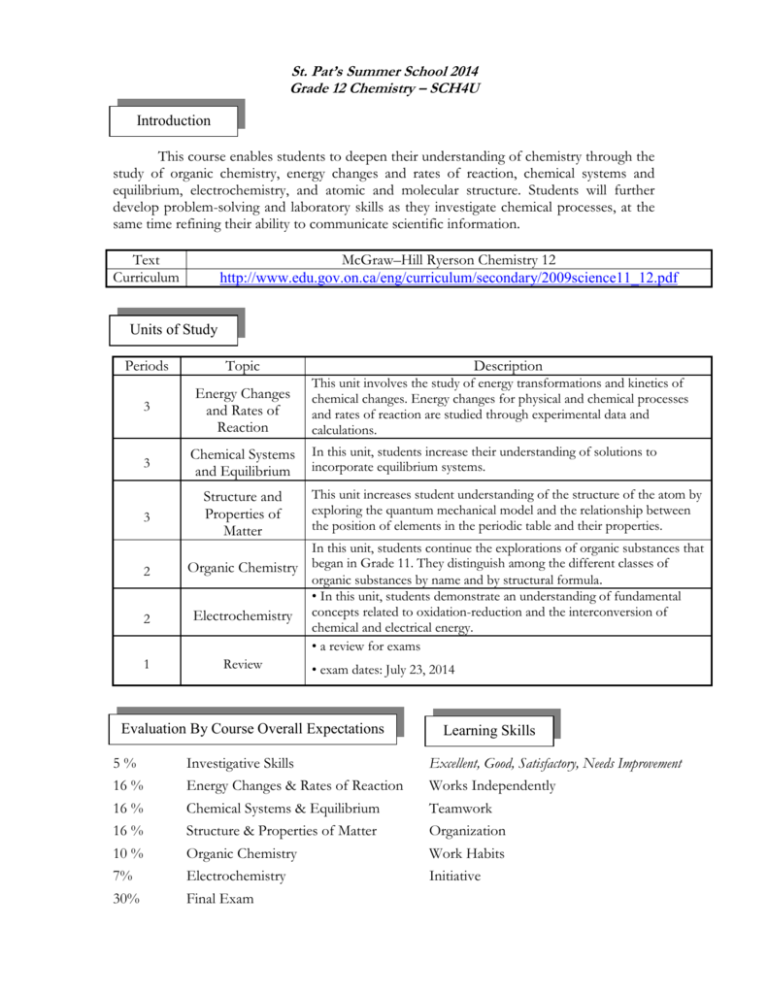
St. Pat’s Summer School 2014 Grade 12 Chemistry – SCH4U Introduction This course enables students to deepen their understanding of chemistry through the study of organic chemistry, energy changes and rates of reaction, chemical systems and equilibrium, electrochemistry, and atomic and molecular structure. Students will further develop problem-solving and laboratory skills as they investigate chemical processes, at the same time refining their ability to communicate scientific information. Text Curriculum McGraw–Hill Ryerson Chemistry 12 http://www.edu.gov.on.ca/eng/curriculum/secondary/2009science11_12.pdf Units of Study Periods Topic 3 Energy Changes and Rates of Reaction 3 Chemical Systems and Equilibrium 3 Structure and Properties of Matter 2 2 1 Description This unit involves the study of energy transformations and kinetics of chemical changes. Energy changes for physical and chemical processes and rates of reaction are studied through experimental data and calculations. In this unit, students increase their understanding of solutions to incorporate equilibrium systems. This unit increases student understanding of the structure of the atom by exploring the quantum mechanical model and the relationship between the position of elements in the periodic table and their properties. In this unit, students continue the explorations of organic substances that Organic Chemistry began in Grade 11. They distinguish among the different classes of organic substances by name and by structural formula. • In this unit, students demonstrate an understanding of fundamental Electrochemistry concepts related to oxidation-reduction and the interconversion of chemical and electrical energy. • a review for exams Review • exam dates: July 23, 2014 Evaluation By Course Overall Expectations 5% 16 % 16 % 16 % 10 % 7% 30% Investigative Skills Energy Changes & Rates of Reaction Chemical Systems & Equilibrium Structure & Properties of Matter Organic Chemistry Electrochemistry Final Exam Learning Skills Excellent, Good, Satisfactory, Needs Improvement Works Independently Teamwork Organization Work Habits Initiative Evaluation Policy It is the responsibility of the student to complete homework when assigned and to catch up on any missed work. For any predictable absence (e.g. dentist appointment, sports event, field trip) from any evaluation other than a test or presentation, alternate arrangements must be made with the teacher prior to the day of the evaluation. Tests must be written on the scheduled day. Any student missing a test for a legitimate reason must provide a parental note or a medical certificate detailing the reason for the absence. A missed test will be given an incomplete unless a note is received within two (2) days of the absence. Regular quizzes will be given. Students who miss a quiz may be given a quiz at the teacher’s discretion. Students are expected to submit completed work on time. If late assignments occur regularly, contact with parents will be taken. Once work has been returned to the class, or taken up by the teacher, the assignment will not be accepted and the student may be asked to complete an alternate assignment. Lates to class will be recorded. Chronic lates may result in detention time, a phone call home or a visit to the office. Academic dishonesty is "cheating or attempting to cheat by using unauthorized material when writing an exam, completing an assignment, or completing any other form of evaluation". A student involved in academic dishonesty will immediately be referred to the Vice-Principal for disciplinary action. Required Materials Every day, students will bring to class: textbook 3 ring binder with four dividers pencil/ pen eraser calculator ruler paper separate notebook Textbook Information The textbook we will be using is McGraw–Hill Ryerson Chemistry 12. The replacement cost is $85.00.











