Lecture II_Crystallinity
advertisement
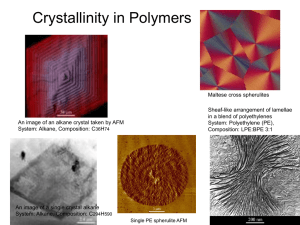
Summary: Last week • Different conformations and configurations of polymers • Molcular weight of polymers: – Number avarage molecular weight (Mn) – Weight average molecular weight (Mw) – Viscocity average molecular weight (Mv) – Polydispersity (PDI) Summary: Methods to analyze different molecular weights • Primary (absolute values) methods – Osmometry (Mn) – Scattering (Mw) – Sedimentation (Mz) Z-average molecular weight is obtained from centrifugation data • Secondary (relevant to reference or calibration) methods – Gel permeation chromatography (GPC) / size exclusion chromatography (SEC) to obtain molecular weight distribution – Intrinsic viscosity for determining viscosity average molecular weight Solid state of polymers Amorphous Crystalline Elastomers, fibers, plastics • Mechanical properties of polymers can be tailored by appropriate combinations of crystallinity, crosslinking and thermal transitions, Tg and Tm • Depending on the particular combination, a specific polymer will be used as a fibre, flexible plastic, rigid plastic or elastomer (rubber) • The operating temperature of polymers is defined by transition temperatures Glass transition temeprature (Tg) and melting temperature (Tm) • The glass transition temperature, Tg, is the temperature at which the amorphous domains of a polymer take on characteristic glassystate properties; brittleness, stiffness and rigidity (upon cooling) • Tg is also defined as the temperature at which there is sufficient energy for rotation about bonds (upon heating) • The melting temperature, Tm, is the melting temperature of the crystalline domains of a polymer sample • The operating temperature of polymers is defined by transition temperatures Crystallinity in polymers • Crystallinity depends on the molecular structure of polymers • No bulk polymer is completely crystalline • In semi-crystalline polymers, regular crystalline units are linked by un-orientated, random conformation chains that constitute amorphous regions • Presence of crystalline structures has a significant influence on physical, thermal and mechanical properties – Highly crystalline: polyolefins – Totally amorphous: atactic PS and PMMA Polymer structures A: Linear, amorphous B: Linear, semi-crystalline C: Branched, amorphous D: Slightly cross-linked E: Cross-linked F: Linear ladder structure Crystallinity Melting temperature of crystalline structures, Tm Crystallinity models Folded lamella structure: Fringed-micelle structure: Synthetic polymers are found to crystallize such that the macromolecules fold Suitable for natural polymers such as cellulose and proteins that consist of fibrils Crystalline state: Ordering of polymer chains • Some polymers can organize into regular crystalline structures during cooling from the melt or hot solution • The basic unit of crystalline polymer morphology is crystalline lamellae consisting of arrays of folded chains. Thickness of typical crystallite may be only 100 to 200 Å (10 to 20 nm) – Even the most crystalline polymers (like HDPE) have lattice defect regions that contain unordered, amorphous material • Crystalline polymers exhibit both: – A Tg corresponding to amorphous regions – A crystalline melting temperature (Tm) at which crystallites are destroyed and an amorphous, disordered melt is formed Crystallinity Adjacent re-entry Non-adjacent re-entry Re-entry of each chain in the folded structure can be adjacent or non-adjacent Crystallinity • Ordinary tie-molecules bond two crystalline parts together across the amorphous part. Two chains can also be entangled together by a physical bond (entanglement) Partly crystalline polymers - thermal transitions Crystallinity • A polymer’s chemical structure determines whether it will be crystalline or amorphous in the solid state • Symmetrical chain structures favor crystallinity by allowing close packing of polymer molecules in crystalline lamellae – Tacticity and geometric isomerism (i.e. trans configuration) favor crystallinity – Branching and atacticity prevent crystallization Crystallinity and the effect of hydrogen bonding • Specific interactions (hydrogen bonding between chains) enhance crystallinity • Within nylons, hydrogen bonding between; – Amide carbonyl group on one chain – Hydrogen atom of an amide group of another chain Conformation and configuration of polymer chains in the lamellae • For many polymers, the lowest energy conformation is the extended chain or planar zig-zag conformation (for example PE, polymers capable of hydrogen bonding) • For polymers with larger substituent groups, the lowest energy conformation is a helix (for example in PP, three monomer units form a single turn in the helix) Packing • The extent to which a polymer crystallizes depends on: – Whether its structure is prone to packing into the crystalline state – The magnitude of the secondary attractive forces of the polymer chains • Packing is facilitated for polymer chains that have: – Structural regularity – Compactness – Streamlining – Some degree of flexibility • This means strongly anisotropic materials (directionally dependent) Packing rules • Notation system in crystallography for planes and directions in crystal lattices • Miller index in cubic Polymer chain Polymer structure hierarchy a,b,c – dimensions of crystal lattice Thickness of lamellae W = width l = thickness Lamellae (crystal) Spherulite PE sheet Packing i.e. different crystal structures • Crystallization from concentrated solution: – Single crystals – Twins – Dendrites – Shish-kebab • Melt crystallization: – Micelles – Spherulites – Cylindrites Schematic models of polymer crystallites Spherulite Flow-induced oriented morphologies i.e. Shish kebab Spherulites • Following crystallization from the melt or concentrated solution, crystallites can organize into spherical structures called spherulites • Each spherulite contains arrays of lamellar crystallites that are typically oriented with the chain axis perpendicular to the radial (growth) direction of the spherulite • Anisotropic morphology results in the appearance of a characteristic extinction cross (Maltese cross) when viewed under polarized light Spherulite nucleation and growth • • • • Formation of nuclei Accelerated crystallization: spherulites grow in radius Crystallization slows: spherulites begin to touch each other Crystallinity may still increase very slowly Structural organization within a spherulite in melt-crystallized polymer (Odian, 4th ed., p. 27). Growth of the spherulites • At t0 the melt begins to cool • At t4 the sample is full of spherulites Illustrations of spherulite growth • Film of formation of spherulites: http://www.youtube.com/watch?v=130sUnjUxmQ • More general information regarding formation of spherulites: https://www.e-education.psu.edu/files/matse081/animations/lesson08/u08_morphF.html Nucleation • Crystallization starts via nucleation and continues via crystallite growth • Homogeneous or heterogeneous: – Homogeneous nuclei are formed from molecules or molecular segments of the crystallizing material itself; called spontaneous or thermal nucleation – Heterogeneous nucleation is caused by the surface of foreign bodies in the crystallizing material such as dust particles or purposely added nucleating agents • Crystallization generally occurs only between the Tg and Tm and the crystallization rate passes through a maximum Crystallization kinetics • The extent of crystallization during melt processing depends on the rate of crystallization and the time during which melt temperatures are maintained • Some polymers that have low rates of crystallization (i.e. PCL) can be quenched rapidly to achieve an amorphous state • On the other hand, some polymers crystallize so rapidly that a totally amorphous state cannot be obtained by quenching (PE) Crystallization kinetics • Fractional crystallinity (X) - Johnson, Mehl, Avrami: X (t ) 1 exp kt m X(t) = fractional crystallinity k = temperature dependent growth rate parameter m = nucleation index, temperature independent • Nucleation • Growth of crystals Rate of crystallization Crystallization rate: Effect of temperature Linear growth of spherulites in PET as a function of temperature (pressure 1 bar) • Tg = 69°C and Tm = 265°C for PET • The maximum growth rate is observed near 178°C. Thermal transitions Melting Thermal transitions • Generally affected in the same manner by: – Molecular symmetry – Structural rigidity – Secondary attractive forces of polymer chains • High secondary forces (due to high polarity or hydrogen bonding) lead to strong crystalline forces requiring high temperatures for melting • High secondary forces also decrease the mobility of amorphous polymer chains, leading to high Tg Melting temperature of polymers • Loss of crystalline structure causes many changes in properties when a material changes into viscous fluid • Polymer melting takes place over a wide temperature range due to the presence of different sized crystalline regions and the complicated process for melting large molecules • Changes in various properties can be used to measure Tm: • • • • • Density Refractive index Heat capacity Enthalpy Light transmission Effect of molecular weight on melting temperature • Dependence of Tm of PLLA and molecular weight at high molecular weights is expressed with Flory equation: 2 RM 0 1 / Tm Tm H m M n Tm = melting temperature at high molecular weights (K) Tm = melting temperature (K) R = gas constant (8.314 J/(molK)) M0 = molecular weight of the monomer (g/mol) Hm = melting entalphy (J/mol) Main chain flexibility Ethyleneglycol-based polyesters Melting temperatures of crystallites and heat treatment Degree of crystallinity in PE at different temperatures Melting temperature of polymer crystallites and effect of heat treatment • Gibbs-Thompson formula connects the melting temperature and the lamellar thickness (L) Tm T 1 2 / Hm L Tm = melting temperature of a lamellar with thickness L T = melting temperature of a infinitely thick and complete crystallite (414.2K) = free surface energy per unit area (79 x 10-3 J / m2) Hm = Enthalpy change per volume (288 x 106 J / m3) L = lamellae thickness ( ) = typical values for PE Effect of crystallinity on properties • In most common semi-crystalline thermoplastic polymers, the crystalline structure contributes to the strength properties of the plastics – Crystalline structures are tough and hard and require high stresses to break them • Mechanical properties of semi-crystalline polymers are mostly dependent on the average molecular weight and degree of crystallinity • Crystallinity affects the optical properties – The size and structure of crystallites Effect of crystallinity on properties PE crystallinity as a function of molecular weight Fragile wax ductile wax Crystallinity % Hard plastic Soft wax Oily Soft plastic Amorphous state Glass transition temprerature, Tg Amorphous state • Completely amorphous polymers exist as long, randomly coiled, interpenetrating chains • Chains are capable of forming stable, flow-restricting entanglements at high molecular weight: – In the melt, long segments of each polymer chain moves in random micro-Brownian motions – As the melt is cooled, a temperature is reached at which all long range segmental motions cease (glass transition temperature, Tg) • In the glassy state, at temperatures below Tg, the only molecular motions that can occur are short range motions i.e. secondary relaxations Critical molecular weight • The minimum polymer chain-length or critical molecular weight Mc for the formation of stable entanglements depends on the flexibility of polymers chain • Relatively flexible polymer chains (such as PS) have a high Mc while more rigid chain polymers (with an aromatic backbone) have a relatively low Mc • Typically, the molecular weight of most commercial polymers is significantly greater than Mc in order to have maximum thermal and mechanical properties • Molecular weight of a commercial polymer is typically 100 000 to 400 000 g/mol while the Mc is only about 30 000 g/mol Theories on glass transitions • Glass transition is at least a partially kinetic phenomenon • The experimentally determined value varies significantly with the timescale of the measurement • Free volume theory: – Glass transition temperature is the temperature with a certain free volume. Many polymers follow the William-Landel-Ferry (WLF) equation • Kinetic theories: – Free volume disappears following kinetic that can be correlated with temperature with Arrhenius. At glass transition the relaxation times are about the same magnitude as measuring times Theory • Williams-Landels-Ferry (WLF) equation: log aT aT C1 T Ts C2 T Ts T T T T s Universal approximation for values of C: 17.44 T Tg log s 51.6 T Tg A = viscosity = characteristic relaxation time of the segments at T and Ts (Ts reference) Ci = empirical constants Effects of the structure on Tg • Glass transition temperature is affected by: – – – – – Polar, intermolecular forces increase Tg Bulky side groups increase Tg Syndiotacticity increases Tg Trans-isomers have higher Tg than cis-isomers Main chain flexibility lowers Tg Molecular structure • Rigidity of polymer chains is especially high when there are cyclic structures in the main polymer chains – Polymers such as cellulose have high Tg and Tm values • Polymers with rigid chains are difficult or slow to crystallize, but the portion that does crystallize will have a high Tm • The extent of crystallinity can be significantly increased in such polymers by mechanical stretching to align and crystallize the polymer chains Main chain structure • Ring structures or unsaturated chemical bonds in the polymer backbone stiffen the chain structure and increase the Tg • Strong polar interactions increase the glass transition temperature – Side groups in polyacrylnitrile are not large but due to polarity Tg is (104 °C) Effect of the backbone on glass transition • Polyethylene • Poly(ethylene oxide) • Poly(dimethylsiloxane) • Poly(ethylene terephtalate) • Polycarbonate Substituents: Tg of substituted vinyl polymers Fried, 2nd ed., p. 179 Side chain: Tg of di-substituted vinyl polymers Fried, 2nd ed., p. 179 Effect of molecular weight on glass transition temperature • For many polymers, Tg increases as average molecular weight increases until a limiting value. After this any further increase in molecular weight does not increase the Tg • Fox-Flory equation can be used to estimate the dependence of Tg on molecular weight: Tg Tg K Mn Tg =the limiting value of T at high molecular weight g K = constant for a given polymer M n = number average molecular weight • K is not constant for molecular weights below 10 000 g/mol Effect of branching on Tg • Branches lower the glass transition temperature which is mainly due to the increased number of end groups • Poly(vinyl acetate) • Highly branched Tg = 25.4 °C • Only few branches Tg = 32.7 °C Effect of crosslinking on glass transition • Long range segmental motion is restricted by crosslinking, thus crosslinking elevates the glass transition temperature – Tg increases with an increase in the degree of crosslinking • Note! Extensive crosslinking causes high chain rigidity which completely prevents crystallization Effect of plasticizer on Tg • Tg of a good plasticizer needs to be lower than the Tg of the polymer • Inverse rule of mixtures (Fox equation when applied to Tg): 1 w1 w2 Tg TgP Tg1 Tg = glass transtion temperature of the composition Tgp = glass transition temperature of the polymer Tg1 = glass transition temperature of the plasticizer w1 = weight fraction of the polymer (%) w2 = weight fraction of the plasticizer (%) Co-polymers and polymer blends • Co-polymer is usually softer than its homopolymers and the Tg is lower • Blends: – A mixture of two homopolymers has two glass transition temperatures near the temperatures of the homopolymers – The miscibility of the blend affects the transitions Simple rule of mixture for binary mixture (polymer blends) Tg=W1Tg,1+W2Tg,2 • W1 is the weight fraction and Tg1 (in Kelvins) the glass transition temperature of the component 1 • Good approximation for blends of two or more polymers but overpredicts the Tg when one component is a low molecular weight organic compound Tg for copolymers of e-caprolactone and D,Llactide • Tg for pure, semi-crystalline polycaprolactone is about -60ᵒC and melting temperature (Tm) about 60ᵒC • Tg for amorphous P(D,L-LA) is 5057ᵒC Wada, R. et al., In vitro evaluation of sustained drug release from biodegradable elastomer, Pharmaceutical research , 8 (1991) 1292-1296 Glass transition in P(CL-DL-LA) co-polymers depending on the monomer ratio The amount of caprolactone in the monomer feed (%) Glass transition in styrene-ethyleneacrylate co-polymers depending on the monomer ratio The amount of styrene (%) Second order transitions • A first order transition is defined as one for which a discontinuity occurs in the first derivative of the Gibbs free energy – In polymers, the first order transition occurs as discontinuity in volume and thus crystalline-melting temperature is such a transition (Tm) • Tg is a second order transition involving a change in the temperature co-efficient of the specific volume and a discontinuity in specific heat • The value of glass transition measured depends on the method and the rate of the measurement Parameters affecting glass transition temperature: summary Polymer based: Processing based: • Chain stiffness: • • • • • • • • • • Structure of the backbone Side groups and branching Stereoregularity Crosslinking Copolymers • Intra- and intermolecular secondary interactions • Average molecular weight • Degree of crystallinity Plasticizers and solvents Blends Fillers Orientation Rate of cooling Next week: • Methods to measure thermal transitions: – TGA – DSC – DMA • Structure characterization: – FTIR – NMR