Notes for Introduction and chapter 1

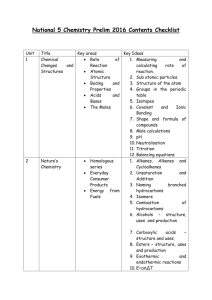
BIOCHEMISTRY
I. INTRODUCTION
Originally, processes in living organisms were thought to be fundamentally
1 different from the processes in non-living material.
This “vital theory” was first discredited in 1828 by Friedrich Wohler.
Wohler synthesized urea (organic) from ammonium cyanate(inorganic).
It is now well-established that there is no fundamental difference between the chemicals or reactions in living versus non-living systems.
Biochemistry is the study of living systems at the molecular level; the study of the chemistry of life processes.
Aim to understand life in molecular terms, including:
chemical structures
physical and chemical properties
reactions, interactions and organization
energy considerations
We will divide our study into 3 main areas:
structural chemistry– chemical structures, functional groups,
3-dimensional structures, relationship of structure function,
catalysts and their kinetics
metabolism – reactions involved in living organisms
molecular genetics – substances and processes involved in the biological transfer of information
There are 4 main classes of biochemicals:
proteins – polymers of amino acids muscles, collagen, hair, enzymes, transport, etc.
carbohydrates – “sugars” (mono-, oligo- and polysaccharides) energy storage (glucose; blood sugar), structure (cellulose)
lipids – fats, oils, membranes and steroids energy storage, form cell membranes, lubrication and protection, waxes, hormones and vitamins or their precursors
nucleic acids– DNA and RNA (polymers of nucleotides) information transfer, direction of protein synthesis, storage and release of energy (ATP ADP)
2
Polymers
Polymeric forms are important for proteins, carbohydrates, nucleic acids.
Polymer – very large molecule consisting of smaller similar subunits
(monomers) which are joined covalently.
Classification of polymers by monomer type: homopolymer – all monomers identical (one type of monomer) heteropolymer – 2 or more types of monomers are present
Classification of polymers by overall structure:
3 linear polymer – monomers are joined sequentially (train cars) branched polymer – has side chains (like tree branch) network polymer(gels) – 3-d interconnections (web)
Biochemistry is a subclass of organic chemistry (carbon compounds).
Why carbon?
light element that forms relatively strong covalent bonds
great capacity to bond to itself and strongly to several other elements (see figure 1.1)
>99% of mass of living organisms from C,O,H,N with S and P
next most important
number of ways to bond is great (isomerism)
tetrahedral bonding pattern of C allows for many types of shapes
4
II. REVIEW
General chemistry:
1.
Bonding a.
ionic and covalent (also electronegativity and polarity) b. single, double and triple bonds covalence – normal number of bonds formed by atoms of a given
element
C = 4 H = 1 N = 3 O, S = 2 c.
Lewis electron dot structures (how to draw)
2.
Acid-Base Chemistry a.
self-ionization of water b.
ionization of acids and bases c.
titration curves d.
pH, pK w
, pK a
, pK b
3.
Molecular Geometry a. linear, bent, trigonal planar, tetrahedral
4. Kinetics a.
zero, first and second order b.
rate equations from mechanistic steps
4.
Equilibria a. K eq
expressions
5.
Thermodynamics a.
enthalpy, entropy and free energy b.
activation energy and energy diagrams
6.
Intermolecular forces (hydrogen bonding, Van der Waal's forces)
5
Organic chemistry:
1. Functional groups (see page 7)
Alcohol, Thiol, Aldehyde, Ketone, Carboxylic acid, Amine (primary, secondary, tertiary), Amide, Ester, Ether, Hemiacetal, Acetal,
Hemiketal, ketal, Phosphate ester
2. Reactions (study these first, others will be mentioned as needed)
Synthesis of amides, esters, hemiacetals and hemiketals
3.
Stereochemistry a.
definitions - enantiomers, diastereomers, optical activity, etc. b.
chirality in reactions
Other review:
1.
General biology a.
Components of prokaryotic and eukaryotic cells b.
cell division cycle
2.
Mathematics a.
general algebra b.
logarithms (base 10 and natural logs) c.
Metric system
6