Introduction Presentation
advertisement

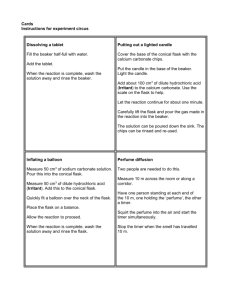
+ Chemistry Year 10 2015 + Conservation of Mass in Experiments + Conservation of Mass Prac 1 Aim: To investigate if mass is conserved in a chemical reaction. Method A: 1. Add 20mL of Vinegar to the conical flask and weigh. Record your weight. 2. Weigh 2.0g of sodium bicarbonate on a watch glass. 3. Add the sodium bicarbonate to the conical flask and swirl until the bubbling stops. Weight the flask and record the weight. + Conservation of Mass Results A: Prediction: Initial Weight of Conical flask and vinegar: Initial Weight of sodium bicarbonate: Total initial weight: Total final weight: + Conservation of Mass Prac 2 Aim: To investigate if mass is conserved in a chemical reaction. Method B: 1. Add 20mL of Vinegar to the conical flask and weigh it with the balloon. Record your weight. 2. Weigh 2.0g of sodium bicarbonate on a watch glass. 3. Add the sodium bicarbonate to the conical flask and quickly cover the opening of the flask with a balloon. Swirl until the bubbling stops and weigh the flask and record this weight. + Conservation of Mass Results B: Prediction: Initial Weight of Conical flask and vinegar: Initial Weight of sodium bicarbonate: Total initial weight: Total final weight: + Discussion Questions Compare the initial and final masses of each part of the experiment. Explain any differences. How does this experiment support the Law of Conservation of Mass? + Experiment 3 – Precipitation Reactions + Precipitation Reactions – Prac 3 1. For each of the precipitates formed in the table above. a. Identify the ions that have formed the precipitate. b. Write the formula of the precipitate formed. c. Write a word equation for the reaction. 2. Write balanced formula equations of the reactions between 3. Silver nitrate (AgNO3(aq)) and sodium chloride (NaCl(aq)) 4. Iron(III) chloride (FeCl(3)(aq)) and sodium hydroxide (NaOH(aq)) 5. Why is it important not to touch the tip of the dropper bottle to the top of the solution in the well. + Conservation of Mass Aim: To investigate if mass is conserved in a chemical reaction. Method: 1. Add 20mL of ?? ?? to a beaker and record the weight of the beaker and solution. 2. Tare (or zero) your scales with a beaker on them, then add 20mL of ?? ?? Record the mass of the solution. 3. Add the second chem to the first, observe the reaction then record the mass of the products. + Conservation of Mass Results: Prediction: Initial Weight of beaker and ?? ??: Initial Weight of ?? ??: Total weight of reactants: Total weight or products: + Discussion Questions 1. Compare the initial and final masses of each part of the experiment. Explain any differences. 2. How does this experiment support the Law of Conservation of Mass? 3. Is this reaction an example of a decomposition, combination or precipitation reaction? How do you know? 4. The chemical formed in this experiment is ??? ??? Use this information to write a. A word equation for this reaction. b. A formula equation for this reaction.