Annex C
advertisement

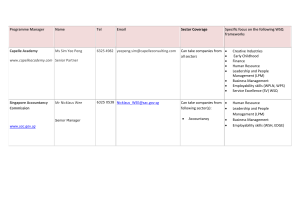
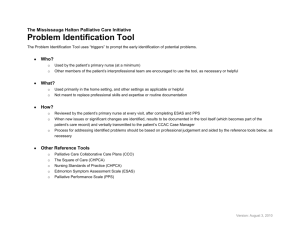
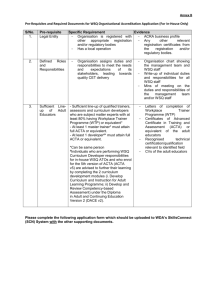
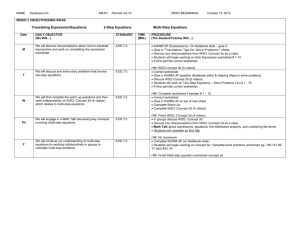
Annex C 3-YEAR PROJECT PROPOSAL OUTLINE For the appointment of CET Centres and Programme Partners for the Clinical Research Workforce Skills Qualifications (WSQ) Framework Interested Training Providers are invited to submit a proposal detailing the following: a) b) Organizational & Corporate Governance Structure i. Registration with ACRA ii. Board of Directors, their roles and written profile of each member iii. Mission & vision iv. Organizational chart, constitution, organisation size and turnover v. Short write-up on the management’s roles and responsibilities in translating the corporate goals into operating plans that guide and facilitate day-to-day activities and tasks vi. Listing of subsidiaries associated / affiliated to organisation vii. Compliance with local regulatory bodies (e.g. Council for Private Education, ACRA) viii. Significant achievements & awards in areas such as EduTrust Certification Scheme, Business Excellence Schemes, International Organization for Standardization ix. System & processes to ensure training quality including a culture of continuous improvement Training Facility, Capacity & Curriculum i. CETCs must have an existing training capacity and evidence of capacity to deliver at least 400 training places over 3 years; PPs must have a training capacity of at least 300 training places over 3 years and show evidence of the plans put in place to achieve this. ii. CETCs and PPs must have evidence of capability to operate and manage a training institution that accepts general public registrations, other than corporate clientele. iii. CETCs and PPs must possess adequate training and assessment facilities (please provide details such as location, training capacity, number of classrooms and set-up including photographs). iv. CETCs and PPs must provide information on current repertoire of course offerings: range & variety, courseware/programme designs, course duration, course fee, frequency of course intakes and number of trainees trained. Page C1 Annex C c) 1 2 v. CETCs and PPs must also show evidence of innovative training methodologies including the use of blended learning (which may include but not limited to elearning based training); use of home-based learning materials; and flexible class scheduling particularly for adult workers with erratic work schedules. vi. CETCs and PPs must show capabilities in developing WSQ professional documents. These documents will include WSQ Competency Standards (CS), WSQ Standard Assessment Plans (SAP) and WSQ Curriculum Training and Assessment Guides (CTAG). Credentials, Experience and Track Record i. CETCs and PPs should possess the domain knowledge, experience, qualifications and expertise needed to facilitate and deliver the Clinical Research WSQ programmes effectively to the relevant target audience. Please provide a current list of accredited Clinical Research WSQ courses, including training achievements over the last 2 years outlined in Annex C – Appendix I (if applicable) ii. CETCs and PPs should possess evidence of having at least 2 years of experience in offering training related to Clinical Research or other related training. Please provide a current list and track record of conducting such nonClinical Research WSQ courses and training achievements over the last 2 years (if applicable) iii. CETCs and PPs should provide a record of their corporate clientele and market penetration, specifying details on the respective organisation profiles and industry sectors. CETCs and PPs should also provide a record of their profile of trainees. iv. CETCs and PPs should have the ability to conduct Training Needs Analysis in order to recommend appropriate training courses to companies to bridge skill gaps in their organisation and also have the experience in developing and implementing training support systems. v. CETCs should provide credentials of the Joint Committee members (cochairman & members). vi. CETCs and PPs should provide credentials of the team members in charge of implementing the programme. vii. CETCs and PPs should provide credentials and curriculum vitae of your Adult Educators (trainers, assessors and curriculum developers) who will be developing and delivering WSQ courses under Clinical Research WSQ. Please also indicate the number of ACTA1 or DACE2-certified Adult Educators in the team and whether these are full-time, part-time employees or adjunct faculty. viii. Any other credentials or proven track record that supports the delivery of WSQ courses. ACTA: Advanced Certificate in Training and Assessment DACE: Diploma in Adult and Continuing Education Page C2 Annex C d) e) Industry Support, Marketing Outreach and Branding Strategy i. CETCs and PPs should provide detailed business strategy including the organisation’s value proposition in delivering courses under Clinical Research WSQ, competitive advantage in the sector, expansion plans – local and/or overseas (if any) and on-going or future collaboration plans with local/foreign institutions and/or partners. ii. CETCs and PPs should provide a detailed industry scoping and analysis of the Clinical Research industry incorporating various sectors, where applicable. CETCs and PPs should provide recommendations on how they would be able to meet the manpower needs of the local Clinical Research industry in relation to Clinical Research WSQ. iii. CETCs and PPs should provide detailed marketing outreach and branding plan to engage employers and individuals from manufacturing industry to increase enrolment into WSQ courses. The plan should include details on outreach plans to target various segments in the industry such as new entrants and existing workers, employer-sponsored and self-sponsored individuals, as well as for different occupational levels and target groups (SMEs and MNCs), and penetrate at least one third of the Clinical Research workforce. iv. CETCs and PPs should provide details on best-in-class tie-ups and proposed collaborations e.g. best-in-class masterclasses, WSQ articulation, reverse engineering of internationally recognised certifications. v. CETCs and PPs should provide projected number of training headcounts and SOAs and a demand analysis from potential companies. vi. CETCs and PPs should provide information on any collaboration and extent of collaboration with industry, agencies, associations and external parties. Learner Records Management & Career Services Plan i. CETCs and PPs should provide details on learner support and administration system for monitoring post training outcomes. The system should capture preenrolment screening, trainees’ registration details, trainees’ attendance, course fees paid, training outcomes, issuance of SOAs/Certificates, employment status before and after training and any other information. ii. CETCs and PPs should have in place procedures to maintain confidentiality of learner records. iii. CETCs should provide evidence of successful career services intervention, career facilitation and professional development services for trainees, such as, seminars, workshops for trainees to be trained in interview skills and resume writing; career advice and talks; organize recruitment seminars and fairs; linking up with companies or industries to offer job placement opportunities for trainees, according to the format in Annex C: Appendix II. iv. CETCs should provide career services and employment facilitation strategy plan with government agencies and industry partners i.e. Train and Place, Page C3 Annex C Place and Train Apprenticeship, Management Associate Programme, Mature workers mentorship programme, tie-ups with SCORE, Back-to-work women strategy, etc. f) Financial Practices & Business Viability CETCs and PPs should provide evidence of company’s past 3 years of certified/audited financial statements in accordance to the Singapore Financial Reporting Standards (FRS) and should include: g) i. Income & Expenditure Statement ii. Cash flow statement iii. Balance Sheet Course Fee Pricing and Strategy for modules under Clinical Research WSQ Framework i. CETCs and PPs must provide a quotation of course fees for delivery of Clinical Research WSQ modules (i.e. Levels 1 - 5) as well as the target training places per year over the 3-year appointment period, based on the template provided in Annex C: Appendix III. ii. CETCs and PPs should provide evidence of the targeted course offerings, innovative training design, delivery and/or assessment strategies as stated below: 1. Training and Assessment Pathway Training - Public runs of all modules under Clinical Research WSQ targeted at the PMEs who are interested to upgrade their skills as well as unemployed jobseekers who are interested to join the Clinical Research industry; 2. In-company contextualised training – Contextualised training that addresses the needs of PMEs in individual companies. Contextualisation should be in the form of curriculum change and /or structural change (change in both curriculum and training delivery). The proposal should present a company engagement strategy and number of companies that the CETC(s) / PP(s) intend to reach out to. A list of potential companies should be included where possible; 3. Assessment-only-pathway (AOP) training – Public runs of Clinical Research WSQ modules targeted at the existing PMEs in the Clinical Research industry who are looking for formal recognition of their skills though certification via assessment only pathway; 4. Masterclasses and seminar-based training – Public runs of Clinical Research related training, targeted at PMEs in the Chemicals industry. iii. A comprehensive programme evaluation framework (e.g. Kirkpatrick’s four levels of evaluation) that addresses outcomes for both individuals (selfsponsored and employer-sponsored) as well as organisations. Page C4 Annex C iv. h) CETCs and PPs must also provide a quotation of fees for the development of WSQ professional documents for the Clinical Research WSQ modules (i.e. Levels 1 - 5) as well as a development timeline, based on the template provided in Annex C: Appendix IV. Any other information CETCs and PPs should provide any other details that would be useful for assessing the company’s suitability to undertake the project in reference but not limited to the evaluation criteria as stipulated in this Call for Proposal. All Call for Proposal applications submitted will be reviewed by WDA for suitability. WDA’s decision is final. Page C5 Annex C APPENDIX I LIST OF ACCREDITED CLINICAL RESEARCH WSQ COURSES (where applicable) S/N Eg1. Competency Unit (CU) Title CU Code WSQ Accreditation Approval Date Apply 5S Techniques MF-COM-101C-2 01 Sep 2010 Programme Title (if applicable) WSQ Training Achievements (SOAs) over the last three years Apply 5S Techniques 2010: 50 2011: 300 Page C6 Annex C APPENDIX II TRACK RECORD OF CAREER SERVICES AND CONSULTANCY SERVICES S/N Category Programme Title Description Period Eg 1. Career Services Job placements Employer servicing capabilities to obtain job vacancies to facilitate employment opportunities for self-sponsored or unemployed trainees XXXX Eg 2. Tracking Trainee Tracking Monitoring of trainees’ employment status 3 months after their graduation XXXX Tracking / Career Services / Consultancy Services Page C7 Annex C APPENDIX III PROPOSED COURSE FEES OF CLINICAL RESEARCH WSQ COURSES TO BE DELIVERED S/N WSQ Level Credit Value (CV) Proposed Course Fee (per pax, excl. GST) Proposed training places / SOAs in Year 1 (Nov 12 – Oct 13) Proposed training places / SOAs in Year 2 (Nov 13 – Oct 14) Proposed training places / SOAs in Year 3 (Nov 14 – Oct 13) Grand Total Training Places for 3 years 1-2 1 Higher Certificate 3-4 5 & above 1-2 2 Advanced Certificate 3-4 5 & above 1-2 3 Diploma 3-4 5 & above 1-2 4 Graduate Diploma 3-4 5 & above 1-2 5 Post-Graduate Diploma 3-4 5 & above Page C8 Annex C APPENDIX IV PROPOSED FEES FOR WSQ PROFESSIONAL DOCUMENTS DEVELOPMENT Module Title Eg. Apply Research Methodology & Ethics Document Start Date End Date Man Hours Cost Competency Standard 1 Jan 2013 1 Jun 2013 50 $1000 Standard Assessment Plan 1 Jan 2013 1 Jun 2013 25 $500 Curriculum, Training and Assessment Guide 1 Jan 2013 1 Jun 2013 12.5 $250 Page C9

![013—BD Global [DOC 117KB]](http://s3.studylib.net/store/data/005892885_1-a45a410358e3d741161b3db5a319267b-300x300.png)