File
advertisement

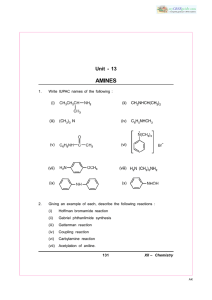
Amines and amides Reactions of Benzene Benzene Know that Benzene has a cyclic structure with formula C6H6 Interpret evidence to support the delocalised model of C6H6 over that of the Kekule model, in terms of bond lengths, electron density and isomers when chlorine is added across a C=C Describe the delocalised structure of Benzene in terms of sigma and pi bonds Use enthalpy of hydrogenation data to support the structure of C6H6 as being Benzene and having delocalisation stability Name aromatic compounds containing Cl, NO2, OH, NH2 and alkyl side groups, using the terms –benzene or phenylExplain why Benzene is resistant to electrophilic addition Explain what is meant by the term electrophilic substitution Explain the use of a halogen carrier e.g. AlCl3, in acylation via a FriedelCrafts reaction and how it achieves this Write an equation for the electrophilic substitution of Benzene with an acyl chloride, using AlCl3 as a halogen carrier Draw a mechanism for the above reaction Write equations to show the formation of a Nitronium ion from concentrated nitric and sulphuric acid Draw a mechanism for the above reaction Explain why the nitric acid is acting as a base and the sulphuric acid is acting as an acid and a catalyst in the above reaction Be able to identify aliphatic amines as 1o, 2o, 3o and 4o (CARE: + charge on 4o amine) and phenylamine as 1o Explain why 1o, 2o and 3o amines can act as bases in terms of the lone pair of electrons and formation of a dative (coordinate) bond Explain why a quaternary ammonium salt cannot act as a base Explain the trend in base strength of a variety of amines Write equations to show how haloalkanes can react with ammonia or amines to make new amines Draw mechanisms for the above reactions Explain why the above reaction is an example of nucleophilic substitution Write an equation for the reduction of a nitrile to form an amine Compare the lab. and industrial conditions for the above reaction Describe how to make an aromatic amine by reducing nitrobenzene Describe the structure of acid amides and N-substituted acid amides Exam ques Revised ): S: Covered AROMATIC CHEMISTRY AND AMINES (: A2 Chemistry – Unit 4