Non-Ideal Reactor Flow & Design: RTD, Segregation, Mixedness
advertisement
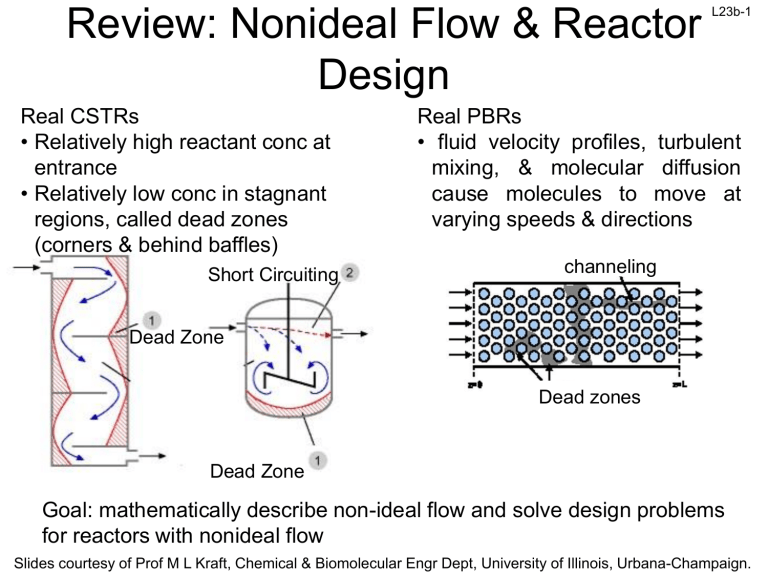
Review: Nonideal Flow & Reactor Design Real CSTRs • Relatively high reactant conc at entrance • Relatively low conc in stagnant regions, called dead zones (corners & behind baffles) Short Circuiting L23b-1 Real PBRs • fluid velocity profiles, turbulent mixing, & molecular diffusion cause molecules to move at varying speeds & directions channeling Dead Zone Dead zones Dead Zone Goal: mathematically describe non-ideal flow and solve design problems for reactors with nonideal flow Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-2 Residence Time Distribution (RTD) RTD ≡ E(t) ≡ “residence time distribution” function RTD describes the amount of time molecules have spent in the reactor RTD is experimentally determined by injecting an inert “tracer” at t=0 and measuring the tracer concentration C(t) at exit as a function of time Measurement of RTD ↑ Pulse injection Et E t dt 1 0 The C curve Reactor Ct 0 C t dt X ↓ Detection C(t) tracer conc at exit between t & t+t sum of tracer conc at exit for infinite time t E(t)=0 for t<0 since no fluid can exit before it enters E(t)≥0 for t>0 since mass fractions are always positive Fraction of material leaving reactor that has t2 E t dt been inside reactor for a time between t1 & t2 t1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-3 E(t) E(t) t Nearly ideal PFR E(t) E(t) t t PBR with dead zones Nearly ideal CSTR Nice multiple choice question t CSTR with dead zones The fraction of the exit stream that has resided in the reactor for a period of time shorter than a given value t: F(t) is a cumulative distribution function 0 E t dt F t t t E t dt 1 F t F t 0 when t<0 0.8 80% of the molecules spend 40 min or less in the reactor F t 0 when t 0 F 1 40 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-4 Review: Mean Residence Time, tm • For an ideal reactor, the space time is defined as V/u0 • The mean residence time tm is equal to in either ideal or nonideal reactors 0 tE t dt tm 0 E t dt 0 tE t dt V tm u0 By calculating tm, the reactor V can be determined from a tracer experiment The spread of the distribution (variance): 2 0 t tm E t dt 2 Space time and mean residence time tm would be equal if the following two conditions are satisfied: • No density change • No backmixing In practical reactors the above two may not be valid, hence there will be a difference between them Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-5 Review: Complete Segregation Model Mixing of different ‘age groups’ at the last possible moment • • • • Flow is visualized in the form of globules Each globule consists of molecules of the same residence time Different globules have different residence times No interaction/mixing between different globules The mean conversion is the average conversion of the various globules in the exit stream: X X t E t t A j A Conversion achieved after spending time tj in the reactor t 0 X A X A t E t dt 0 j j Fraction of globules that spend between tj and tj + t in the reactor XA(t) is from the batch reactor design equation Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-6 Review: Maximum Mixedness Model In a PFR: as soon as the fluid enters the reactor, it is completely mixed radially with the other fluid already in the reactor. Like a PFR with side entrances, where each entrance port creates a new residence time: u0E( ) ∞ 0 u0 u u + V=0 V = V0 : time it takes for fluid to move from a particular point to end of the reactor u: volumetric flow rate at , = flow that entered at + plus what entered through the sides u0E: Volumetric flow rate of fluid fed into side ports of reactor in interval between + & Volumetric flow rate of fluid fed to reactor at : u u0 E d u0 1 F fraction of effluent that in reactor for less than time t Volume of fluid with life expectancy between + & : V u0 1 F Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-7 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for (1) an ideal PFR and (2) for the complete segregation model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-8 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for (1) an ideal PFR and (2) for the complete segregation model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 Start with PFR design eq & see how far can we get: dX A kC A CB2 dV C A0u0 dX A rA dV FA0 dX A kCA0CB0 1 X A dV CA0u0 2 3 CA CA0 1 X A X CB CB0 1 X A 2 V kC A dX A Get like terms B0 dV 3 together & integrate 0 1 X A 0 u0 X A 1 1 2 kCB02 1 1 2kC X 1 B0 V A 2 2 1 X u 2kCB02 1 A 2 1 X A 0 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-9 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for (1) an ideal PFR and (2) for the complete segregation model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 t*E(t) 0 0.02 0.2 0.48 0.8 0.8 0.72 0.56 0.48 0.396 0 XA 1 1 2kCB0 1 2 Use numerical method to determine tm: How do we determine ? 0.3 0.144 For an ideal reactor, = tm tm 0 tE t dt 10 14 0 0 10 tm tE t dt tE t dt tE t dt 1 0 4 0.02 2 0.2 4 0.48 2 0.8 4 0.8 tE t dt 4.57 3 2 0.72 4 0.56 2 0.48 4 0.396 0.3 0 10 14 tE t dt 10 2 0.3 4 0.144 0 0.584 3 tm 4.57 0.584 5.15min Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-10 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for (1) an ideal PFR and (2) for the complete segregation model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 t*E(t) 0 0.02 0.2 0.48 0.8 0.8 0.72 0.56 0.48 0.396 0 XA 1 1 2kCB02 1 X A,PFR 1 0.3 0.144 For an ideal PFR reactor, = tm tm 5.15min tm 0 tE t dt 1 2 L2 mol 2 176 0.0313 5.15min 1 2 L mol min X A,PFR 0.40 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-11 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 Segregation model: X A X A t E t dt XA(t) is from batch reactor design eq 0 Numerical method 1. Solve batch reactor design equation to determine eq for XA 2. Determine XA for each time 3. Use numerical methods to determine X̄A Polymath Method 1. Use batch reactor design equation to find eq for XA 2. Use Polymath polynomial curve fitting to find equation for E(t) 3. Use Polymath to determine X̄A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-12 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 Segregation model: X A X A t E t dt XA(t) is from batch reactor design eq 0 NA0 CA0 V dX A 3 dX A 2 3 dX A 2 kC 1 X kCA0CB0 1 X A V NA0 rA V NA0 B0 A dt dt dt Batch design eq: Stoichiometry: rA kC A CB 2 CA CA0 1 X A CB CB0 1 X A XA 0 dXA t 1 XA 3 1 1 X A 2 kCB02dt 0 X A 1 2 kC t B0 2 2 1 X A 0 1 2kCB02 t XA 1 1 1 2kCB02t Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-13 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 XA Segregation model: X A X A t E t dt XA 1 0 Numerical method XA0 1 X A 1 1 1 1 1 0.3429min1 t 1 2kCB02t Plug in each t & solve 1 1 1 0.3429min 1 0 1 1 0.3429min 1 1min 0 0.137 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-14 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 XA 0 0.137 0.23 0.298 0.35 0.39 0.428 0.458 0.483 0.505 0.525 0.558 0.585 Segregation X A X A t E t dt model: 0 Numerical method XA 1 1 1 1 0.3429min1 t 1 2kCB02t 1 10 14 0 0 10 X A X A t E t dt X A t E t dt X A t E t dt 0 4 0.137 0.02 2 0.23 0.1 4 0.298 0.16 1 X A t E t dt 2 0.35 0.2 4 0.39 0.16 2 0.428 0.12 4 0.458 0.08 3 0 2 0.483 0.06 4 0.505 0.044 0.525 0.03 10 10 X A t E t dt 0.35 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-15 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 XA 0 0.137 0.23 0.298 0.35 0.39 0.428 0.458 0.483 0.505 0.525 0.558 0.585 Segregation X A X A t E t dt model: 0 Numerical method XA 1 1 1 1 0.3429min1 t 1 2kCB02t 1 14 0 10 X A X A t E t dt 0.35 X A t E t dt 14 2 X A t E t dt 0.525 0.03 4 0.558 0.012 0.585 0 0.0425 3 10 X A X A t E t dt 0.35 0.04 X A 0.39 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-16 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 XA 0 0.137 0.23 0.298 0.35 0.39 0.428 0.458 0.483 0.505 0.525 0.558 0.585 Alternative approach: segregation model by Polymath: X A X A t E t dt 0 dXA XA t E t dt XA 1 Need an equation for E(t) 1 1 2kCB02t k=176 CB0=0.0313 Use Polymath to fit the E(t) vs t data in the table to a polynomial Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. time L23b-17 E(t) For the irreversible, liquidphase, nonelementary rxn A+B→C+D, -rA=kCACB2 Calculate the XA using the complete segregation model using Polymath Gave best fit E(t) = 0 at t=0 Model: C02= a1*C01 + a2*C01^2 + a3*C01^3 + a4*C01^4 a1=0.0889237 a2= -0.0157181 a3= 0.0007926 a4= -8.63E-06 Final Equation: E= 0.0889237*t -0.0157181*t2 + 0.0007926*t3 – 8.63E-6*t4 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Complete segregation model by Polymath L23b-18 A+B→C+D -rA=kCACB2 Segregation model by Polymath: XA 0.36 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-19 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 Maximum mixedness model: dXA rA E X d CA0 1 F A rA kCA0CB02 1 XA 3 =time 0 dF E d F() is a cumulative distribution function L2 CA0 CB0 0.0313mol L k 176 mol2 min Polymath cannot solve because →0 (needs to increase) Substitute for z, where z=T̅- where T̅=longest time interval (14 min) E must be in terms of T̅-z. rA dF E T z dX A XA E T z Since T̅-z= & =t, simply dz CA0 1 F T z dz substitute for t E()= 0.0889237*-0.0157181*2 + 0.0007926*3 – 8.63E-6*4 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Maximum Mixedness Model, nonelementary reaction A+B→C+D -rA=kCACB2 dF E T z dz L23b-20 rA E T z dX A XA dz CA0 1 F T z Denominator cannot = 0 z T Tz Eq for E describes RTD function only on interval t= 0 to 14 minutes, otherwise E=0 XA, maximum mixedness = 0.347 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-21 For a pulse tracer expt, C(t) & E(t) are given in the table below. The irreversible, liquidphase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out in this reactor. Calculate the conversion for the complete segregation model under adiabatic conditions with T0= 288K, CA0=CB0=0.0313 mol/L, k=176 L2/mol2·min at 320K, H°RX=-40000 cal/mol, E/R =3600K, CPA=CPB=20cal/mol·K & CPC=CPD=30 cal/mol·K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 dXA dX A 3 2 X t E t kC 1 X Polymath eqs for segregation model: A B0 A dt dt E(t)= 0.0889237*t -0.0157181*t2 + 0.0007926*t3 – 8.63E-6*t4 L2 1 1 exp 3600K 320K T mol2 min Need equations from energy balance. For adiabatic operation: Express k as function of T: k T 176 n T H RX TR X A iCp T0 X A CPTR i i1 n C X C i pi A P i1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-22 For a pulse tracer expt, C(t) & E(t) are given in the table below. The irreversible, liquidphase, nonelementary rxn A+B→C+D, -rA=kCACB2 will be carried out in this reactor. Calculate the conversion for the complete segregation model under adiabatic conditions with T0= 288K, CA0=CB0=0.0313 mol/L, k=176 L2/mol2·min at 320K, H°RX=-40000 cal/mol, E/R =3600K, CPA=CPB=20cal/mol·K & CPC=CPD=30 cal/mol·K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 n Energy balance for T adiabatic operation: n H RX TR X A iCp T0 X A CPTR i i1 cal iCpi CpA CPB 40 mol K i1 cal cal X A 576 mol mol T cal cal 2 XA mol K mol K 1702 n C X C i pi A P i1 Cp 30 30 20 20 dXA XA t E t dt k T 176 Not zero! cal cal 20 mol K mol K dX A 3 kCB02 1 X A dt L2 1 1 exp 3600K 320K T mol2 min E(t)= 0.0889237*t -0.0157181*t2 + 0.0007926*t3 – 8.63E-6*t4 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Segregation model, adiabatic operation, nonelementary reaction kinetics L23b-23 A+B→C+D -rA=kCACB2 XA 0.93 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-24 The following slides show how the same problem would be solved and the solutions would differ if the reaction rate was still -rA=kCACB2 but the reaction was instead elementary: A+2B→C+D These slides may be provided as an extra example problem that the students may study on there own if time does not permit doing it in class. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-25 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 Start with PFR design eq dX A rA dX A kC A CB2 & see how far can we get: dV F dV C A0u0 A0 CA CA0 1 X A 0 dX A kC A CB2 d C A0 b 2 b = = CB CB0 1 2XA a 1 2 dX A kCA0CB0 1 X A 1 2X A d CA0 2 dX A 2 kCB02 1 X A 1 2X A d CB0 = 0.0313 k = 0.0313 Could solve with Polymath if we knew the value of Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-26 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 t*E(t) 0 0.02 0.2 0.48 0.8 0.8 0.72 0.56 0.48 0.396 0 dX A 2 kCB02 1 X A 1 2X A d Use numerical method to determine tm: How do we determine ? 0.3 0.144 For an ideal reactor, = tm tm 0 tE t dt 10 14 0 0 10 tm tE t dt tE t dt tE t dt 1 0 4 0.02 2 0.2 4 0.48 2 0.8 4 0.8 tE t dt 4.57 3 2 0.72 4 0.56 2 0.48 4 0.396 0.3 0 10 14 tE t dt 10 2 0.3 4 0.144 0 0.584 3 tm 4.57 0.584 5.15min Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-27 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.02 0.1 0.16 0.2 0.16 0.12 0.08 0.06 0.044 0.03 0.012 0 t*E(t) 0 0.02 0.2 0.48 0.8 0.8 0.72 0.56 0.48 0.396 0 dX A 2 kCB02 1 X A 1 2X A d 0.3 0.144 For an ideal reactor, = tm tm 0 tE t dt tm 5.15min Final XA corresponds to =5.15 min X A,PFR 0.29 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-28 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 Segregation model X X t E t dt dXA X t E t A A A with Polymath: dt 0 0 XA(t) is from batch reactor design eq Batch reactor dX A 2 2 dX A N k C C 1 X 1 2X V NA0 rA V A0 A0 B0 A A design eq: dt dt NA0 CA0 V dX A 2 Stoichiometry: kCB02 1 X A 1 2X A dt rA kCA CB2 Best-fit polynomial line k=176 CB0=0.0313 CA CA0 1 X A for E(t) vs t calculated by Polymath (slide 19) CB CB0 1 2X A 2 3 4 E(t)= 0.0889237*t -0.0157181*t + 0.0007926*t – 8.63E-6*t Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Segregation model, isothermal operation, elementary rxn: A+2B→C+D L23b-29 XA,seg 0.27 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-30 For a pulse tracer expt, the effluent concentration C(t) & RTD function E(t) are given in the table below. The irreversible, liquid-phase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out isothermally at 320K in this reactor. Calculate the conversion for an ideal PFR, the complete segregation model and maximum mixedness model. CA0=CB0=0.0313 mol/L & k=176 L2/mol2·min at 320K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 dF Maximum mixedness model: dXA rA E X E =time A d d CA0 1 F Polymath cannot solve r kC C 2 1 X 1 2X 2 L2 A A0 B0 A A because →0 (must k 176 mol2 min CA0 CB0 0.0313mol L increase) Substitute for z, where z=T̅- where T̅=longest time interval (14 min) E must be in terms of T̅-z. rA dF E T z dX A XA E T z Since T̅-z= & =t, simply dz substitute for t CA0 1 F T z dz E()= 0.0889237*-0.0157181*2 + 0.0007926*3 – 8.63E-6*4 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Maximum Mixedness Model, elementary reaction A+2B→C+D, -rA=kCACB2L23b-31 dF E T z dz rA E T z dX A X CA0 1 F T z A dz Denominator cannot = 0 z T Tz Eq for E describes RTD function only on interval t= 0 to 14 minutes, otherwise E=0 XA, maximum mixedness = 0.25 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-32 For a pulse tracer expt, C(t) & E(t) are given in the table below. The irreversible, liquidphase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out in this reactor. Calculate the conversion for the complete segregation model under adiabatic conditions with T0= 288K, CA0=CB0=0.0313 mol/L, k=176 L2/mol2·min at 320K, H°RX=-40000 cal/mol, E/R =3600K, CPA=CPB=20cal/mol·K & CPC=CPD=30 cal/mol·K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 Polymath eqs for dXA dX A 2 2 X t E t kC 1 X 1 2X A B0 A A segregation model: dt dt E(t)= 0.0889237*t -0.0157181*t2 + 0.0007926*t3 – 8.63E-6*t4 Express k as function of T: k T 176 L2 1 1 exp 3600K 320K T mol2 min Need equations from energy balance. For adiabatic operation: n T H RX TR X A iCp T0 X A CPTR i i1 n C X C i pi A P i1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L23b-33 For a pulse tracer expt, C(t) & E(t) are given in the table below. The irreversible, liquidphase, elementary rxn A+2B→C+D, -rA=kCACB2 will be carried out in this reactor. Calculate the conversion for the complete segregation model under adiabatic conditions with T0= 288K, CA0=CB0=0.0313 mol/L, k=176 L2/mol2·min at 320K, H°RX=-40000 cal/mol, E/R =3600K, CPA=CPB=20cal/mol·K & CPC=CPD=30 cal/mol·K t min 0 1 2 3 4 5 6 7 8 9 10 12 14 C g/m3 0 1 5 8 10 8 6 4 3 2.2 1.5 0.6 0 E(t) 0 0.16 0.12 0.08 0.02 0.1 0.16 0.2 0.06 0.044 0.03 0.012 0 Adiabatic EB: n H RX TR X A iCp T0 X A CPTR Cp 30 30 2 20 20 0 i i 1 T n cal n iCpi CpA CPB 40 mol K iCpi X A CP i1 i1 T 288K 1000X A dXA XA t E t dt k T 176 dX A 2 kCB02 1 X A 1 2X A dt L2 1 1 exp 3600K 320K T mol2 min E(t)= 0.0889237*t -0.0157181*t2 + 0.0007926*t3 – 8.63E-6*t4 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Segregation model, adiabatic operation, elementary reaction kinetics L23b-34 A+2B→C+D -rA=kCACB2 Because B is completely consumed by XA=0.5 X̅A = 0.50 Why so much lower than before? Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.







