Unit 9E - SciencePBS
advertisement

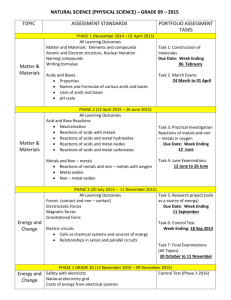
Unit 9E Reactions of Metals Learning Outcome • Describe where to find metals on the periodic table • Describe some properties of metals The periodic table http://www.youtube.com/watch?v=nsbXp64YPRQ Metals and Non-metals Metals and non-metals have very different physical properties. Because of this the position of metals and non-metals show a pattern in the Periodic Table: • Metals are on the left and centre. • Non-metals are mostly on the right • In between are metalloids – these are like metals in some ways and like non-metals in others. Properties of metals Metals are all around us. We use them every day in a variety of forms. With the person sitting next to you create a list of items that you use every day that are made from metals Properties and Uses Metals are used for a variety of reasons because of their many properties. Use the activity to sort out the property of metals with their uses. Property Strength Definition Able to withstand great force Suitability for use bicycle frames, car bodies, bridges Density Mass per unit3 Malleability Able to be shaped by rolling or hammering Low density aluminium in airplane bodies Electrical conductor Ability to conduct an electrical current copper wires in cables Thermal Conductor Ability to conduct a thermal current Pots and pans for cooking car bodies, horse shoes Learning Outcome • State what gas is given off when a metal reacts with an acid. • Describe a test for this gas. Following the instruction card you have been given carry out the experiment. Now discuss the results in your groups. What happened to the light splint? What did you notice about the rates of the reactions when you changed the metal? Copy and complete When some metals react with acid a ________ is released showing that a _________ reaction has taken place. When tested this gas _________ with a _______. pop Burns chemical gas From our experiment the reactivity of the metals is: Learning Outcome • Discover if the same gas is given off with every acid • Describe a test for this gas. • Write a general formula for the reaction of metals with acids. What is the gas and is it the same for every reaction of metal with acid? When you tested some metals with hydrochloric acid a gas was given off. This gas burned with a ‘POP’ Oxygen in the air Lighted spill Mg + acid Look at the chemical equation below magnesium + Mg + hydrochloric magnesium acid chloride HCl MgCl2 From the equation above the gas must be Hydrogen _____________ Will the same gas be given off if we change the acid that we used?? + + hydrogen H2 Carry out the experiment as described on the Experiment work card. What did you discover about the gas released? Draw a labelled diagram to show the experiment you carried out. Reaction with Metals - general • Many metals react with acids to release a salt plus hydrogen gas. • The general word equation is: metal + acid a salt + hydrogen • The salt will depend upon the metal and the acid used. •Hydrochloric acid gives metal chlorides •Sulphuric acid gives metal sulphates •Nitric acid gives metal nitrates •So, for example magnesium + Hydrochloric magnesium acid chloride + hydrogen Use the general equation and the examples below to write the word equations for the reactions you carried out. metal + magnesium + iron calcium Zinc + acid a salt sulphuric acid magnesium sulphate + nitric acid + sulphuric acid hydrochloric acid + hydrogen + hydrogen iron nitrate + hydrogen calcium sulphate + hydrogen zinc chloride + hydrogen Learning Outcome • Describe the products of the reaction between metal carbonates and acids. • Write a general formula for the reaction of metal carbonates with acids. Carry out the experiment as described on the experiment work card. Draw and label a diagram to show what you did. Consider the questions below: 1. What happened to the lime water? 2. What is the gas that is released? 3. What happened to the colour of the original solution? Copy and complete The limewater changed from clear and _________ to _________. This shows the gas produced is _________________. The solution originally was ___________ and changed to ___________ showing that a chemical reaction had occurred. Metal carbonates and acids • When metal carbonates react with acids they fizz giving off carbon dioxide gas. • Most metal carbonates are not very soluble and so reactions may be slow. The general word equation for these reactions is: Metal carbonate + acid a salt + water + carbon dioxide • Now write the word equation for the reaction you carried out. copper carbonate + sulphuric acid calcium chloride + water + carbon dioxide Metal carbonates and acids Metal carbonate + acid a salt + water + carbon dioxide zinc + sulphuric zinc carbonate acid sulphate ZnCO3 + H2SO4 ZnSO4 + water + + H2O + carbon dioxide CO2 sodium + hydrochloric sodium + water + carbonate acid chloride carbon dioxide CO2 Na2CO3 + nickel + carbonate NiCO3 + 2HCl nitric acid 2HNO3 2NaCl nickel nitrate Ni(NO3)2 + H2O + + water + + H2O + carbon dioxide CO2 Learning Outcome • Describe the products of the reaction between metal oxides and acids. • Write a general formula for the reaction of metal oxides with acids. Metal Oxides What are metal oxides? Metal oxides are formed when a metal reacts with oxygen in the air. Metal oxides and acids • Most metal oxides are not soluble. • This means their reaction with acids is often slower. • Heating can help to speed up the reaction. Carry out the experiment as instructed Draw and label a diagram into your jotter Acid What did you notice? Oxide Using the general equation you have been given can you write the word equation for the reaction you have just carried out? Copper oxide + sulphuric acid copper sulphate + water Where is the salt?? From your word equation you have created copper sulphate salt. Acid What could we do to extract the copper sulphate from the water to check our equation? Oxide Metal oxides and acids a salt + water Metal oxide + acid zinc oxide + sulphuric acid zinc sulphate + water ZnO + H2SO4 ZnSO4 + H2O sodium chloride + water 2NaCl + H2O sodium oxide + hydrochloric acid Na2O + 2HCl iron oxide + nitric acid Fe2O3 + 3HNO3 iron nitrate + water 3H2O Fe(NO3)3 + Learning Outcome • Describe what acid rain is and how it is formed • Explain some of the problems associated with acid rain • Describe some methods to reduce acid rain. Your task………. • Create an informative article for a newspaper. You must include – – Pictures – An explanation of what acid rain is – An explanation why acid rain is a problem – Methods of reducing acid rain Learning Outcome • Be able to state what is formed when an acid reacts with metals, metal carbonates and metal oxides. In your teams!! You must create 3 sets of individual cards with: Reactant names Arrows Product names e.g. Hydrochloric acid + Magnesium chloride Magnesium Hydrogen + Once your cards are made: You will pass your cards to the next group. You will be passed cards by another group. You now have 10 minutes to unjumble the cards you have been given and lay out on the desk in front of you the correct answers Now a runner from the group who made your cards will come and assess your answers!! This will be added to the quiz scores!!!! GOOD LUCK! Learning Outcome • Be able to explain what neutralisation is and what the products of neutralisation are. Neutralisation When an acid is added to an alkali or an alkali added to an acid, neutralisation takes place: the substance changes pH to become closer to being neutral. In your group think about examples from everyday life where neutralising an acid or an alkali might be useful. Write your ideas onto a show me board. Alkali Hazards Alkalis just like acids can be dangerous to use. Weak alkalis like calcium hydroxide (lime water) are irritants and can cause skin to redden and itch and blister. Strong alkalis like sodium hydroxide (caustic soda) are corrosive and can cause skin to redden and itch and blister. Learning Outcome • Describe what an ion is. • Explain how salts are formed from ions. Bases Bases are substances that neutralise acids. Bases are usually: •Metal hydroxides •Metal oxides •Metal carbonates contain OH contain O contain CO3 The following general word equation describes neutralisations: acid + base a salt + water In the case of carbonates we also get carbon dioxide. Alkalis Bases are substances that neutralise acids. Alkalis are soluble bases. Although both can neutralise acids solubility is important when it comes to the pH of solutions. For example, adding sodium hydroxide to water gives a solution with a pH of about 14. When calcium carbonate is added to water it does not dissolve and so the pH remains close to 7. Even so it can neutralise acid that is added although more slowly than a soluble base might. Neutralisation - Indigestion If we have too much acid in our stomachs, we get indigestion. Acid can move up out of our stomach creating a burning feeling in the chest. We neutralise the excess acid by taking a tablet containing a base. This is usually a carbonate or an oxide. Strong soluble bases (like sodium hydroxide) would create too alkaline a solution and cannot be used. Acid 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Alkali Neutralisation - Stings A bee sting is acidic. A wasp sting is alkaline. So one way to treat an acidic bee sting is to dab on a base: bicarbonate of soda more properly known as sodium hydrogen carbonate. One way to treat a basic wasp’s sting is with an acid : vinegar - ethanoic acid. Neutralisation – Soil pH Plants remove compounds from the soil in a way that tends to leave the soil acidic. Many plants won’t grow well in acid soil and so farmers have to regularly check the pH and adjust it by adding a base. Calcium carbonate or calcium hydroxide are cheap and so are often used for this purpose. Neutralisation – Soil fertilisers Plants also remove nitrogen compounds from the soil and this is often replaced using fertiliser. Ammonia is a water soluble gas high in nitrogen and in some countries it is injected directly into moist soil. However, because it is a gas much of it is quickly lost. In the UK ammonia is dissolved in water to give ammonium hydroxide (an alkali) and this is neutralised by reacting it with nitric acid to give a solid nitrogen rich fertiliser. ammonium hydroxide NH4OH + nitric acid + HNO3 ammonium nitrate NH4NO3 + water + H2O Neutralisation - acid gases Many power stations burn coal containing sulphur. When this burns it produces acidic sulphur oxides which can cause acid rain. The gases are “scrubbed”, as much as possible, of these acidic oxides by reacting them with a base before releasing them into the air. Calcium oxide or calcium hydroxide are often used for this purpose. Acid rain – living things Steps have been taken to reduce emissions of acidic sulfur oxides from power stations and nitrogen oxides from cars. Even so the atmosphere still contains enough of them to make the rain from industrial areas quite acidic. Trees and lakes are badly affected in many parts of the world including Northern Germany and Scandinavia which suffers from South-West winds from the UK. Acid rain damaged tress Acid rain – metals and stone Acid rain increases the rate of corrosion of metals. It also greatly accelerates the rate of chemical weathering of certain stones used in building such as limestone and marble. (These stones are carbonates. What gas will be given off as they dissolve?) CO2 The metal above the wheel arch of this car is rusting away Learning Outcome • • • • Understand the hazards of alkalis State what a base is State what an alkali is Explain the importance of the solubility of a base in neutralisation Copy and complete Bases are substances that _________ acids. When a base is soluble in water it is called an ___________. An insoluble base will not alter the pH of ________ but will still neutralise an acid although more ________. Alkalis are important in a number of situations e.g. neutralising acidic soil. Learning Outcome • Describe 2 ways to find the exact volume of 1 substance that will neutralise another substance A simple way of neutralising a solution is to add an acid to Am alkali until the indicator turns green. Using a dropping pipette makes it very difficult to measure the exact volume of alkali or acid required for the reaction. Chemists use burettes to add small amounts gradually and to measure volume added accurately. Finding the volume Experiment 1 • Fill the burette with HCl • Accurately measure 25cm3 . of NaOH (using a pipette) into a conical flask • Add 2 drops of universal indicator • Now carefully add HCl from the burette into the conical flask as shown. • Record the volume of HCl needed to neutralise the NaOH. Experiment 2 • Accurately measure 25cm3 of HCl into a beaker • Collect a pH probe and data logger and insert the pH probe into the HCl • Now measure 1cm3 of NaOH and add it into the HCl. • Stir and record the pH • Add another 1 cm3 of NaOH and repeat. • Keep adding until the pH on the data logger reads 7. Graph the results Create a graph of pH of solution against the volume of NaOH added. Using your graph find the exact volume needed to neutralise the HCl. Homework Write a short essay (300 words) on salts and their commercial and industrial importance in society. Learning Outcome • Describe 2 ways to find the exact volume of 1 substance that will neutralise another substance Learning Outcome • Describe 2 ways to find the exact volume of 1 substance that will neutralise another substance Learning Outcome • Describe 2 ways to find the exact volume of 1 substance that will neutralise another substance