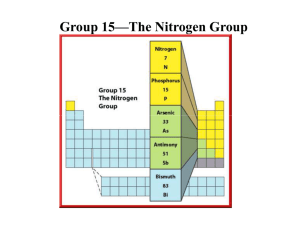
File
advertisement

2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 p-BLOCK ELEMENTS: The elements in which the differentiating electron enters p-sub shell are called p-block elements. Maximum of 6-electrons can be accommodated in a p-sub shell; hence p-block of the periodic table consists of 6-groups. The general electronic configuration of p-block is ns2 np1– 6. The elements belong to the groups from 13 – 18 constitute p-block of the periodic table. GROUP-15 ELEMENTS: The elements nitrogen, phosphorus, arsenic, antimony and bismuth constitute group 15 of the periodic table. They are called as ‘pnicogens’ and their compounds are called ‘pnicomides’. (pniomgs-suffocation). Occurrence: Molecular nitrogen comprises 78% by volume of the atmosphere. Nitrogen occurs as NaNO3 (Chile salt petre), KNO3 (Bengal salt petre) and as proteins in plants and animals. Phosphorus occurs as apatites Ca3(PO4)2.CaX2. Example: Fluorapatite- Ca3(PO4)2.CaF2.Apatites are the main components of phosphate rocks. Phosphorus is present in bones as well as in living cells. Phospho proteins are present in milk and eggs. Arsenic, antimony, and bismuth are found mainly as sulphide minerals. Physical properties of Group 15 elements: Electronic configuration: The general outer most electronic configuration of group 15 elements is ns2np3. Si.No. Element Symbol Atomic number Electronic Configuration 1 Nitrogen N 7 [He]2s22p3 2 Phosphorus P 15 [Ne]3s23p3 3 Arsenic As 33 [Ar]3d104s24p3 4 Antimony Sb 51 [Kr]4d105s25p3 5 Bismuth Bi 83 [Xe]4f14 5d106s26p3 The s orbital of these elements is completely filled and p orbital of these elements is half filled making their electronic configuration extra stable. Atomic radii or Covalent radii: Atomic radii of group 15 elements is less than the corresponding elements of group14. Atomic radii varies as N < P<As < Sb < Bi.That is it increases from N to Bi. The increase in atomic radii is large from N to P due to effective shielding by s and p electrons. There is a small increase in atomic radii from As to Bi due to poor shielding by d and f electrons. Ionic radii: For a given oxidation state ionic radii increases from N to Bi. N –3 <P –3< As–3< Sb –3<Bi–3 and Sb+3 < Bi+3. Ionisation Enthalpy: Ionisation enthalpy of group 15 elements is larger than the corresponding elements of group 14 due to the half filled p-subshell which gives extra stability. Ionisation enthalpy decreases from N to Bi i.e., N > P > As > Sb > Bi. The second and third ionisation enthalpies of Bi are more than antimony. Why? This is due to the involvement of intervening 4f electrons which cause poor shielding effect, hence effective nuclear charge is more than that of Sb and hence it has higher ionisation enthalpy. Electronegativity: These elements possess high electronegativity due to the small size and they need only three electrons to attain ns2np6 configuration. Electronegativity decreases from N to Bi due to increase in atomic size from N to Bi. Metallic and non metallic character: The elements N and P are non-metals As and Sb are metalloids and Bi is a metal. The metallic property increases from N to Bi due the increase in atomic size and decrease in ionisation enthalpy. That increases tendency to lose electrons. Allotropy: Except N and Bi remaining elements will show the phenomenon called allotropy. Phosphorus: White, red, black, scarlet and violet. Chemistry p –BLOCK ELEMENTS Page 1 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Arsenic: Grey, Yellow and black Antimony: Yellow and black. Catenation: Catenation is not noticed in nitrogen to the maximum extent. Nitrogen forms chains extending up to 3 atoms in hydrazoic acid. H – N = N+=N – . In nitrogen two N-atoms are linked to each other by multiple bond N≡N due to high bond dissociation energy. Two nitrogen atoms cannot form N – N single bond due to lone pair-lone pair repulsion between electrons on bonded atoms and size atoms is small. The other family members like P, As, Sb undergo catenation and exists as polyatomic atomic molecules ( P4, As4 and Sb4). They can form P – P, As – As and Sb – Sb single bonds due to their bigger atomic size which reduces repulsion between lone pairs. The elements like P, As and Sb cannot be linked by multiple bonds due to their bigger size and weaker bond dissociation energy. Melting and Boiling points: Generally MP and BP increase from N to Bi. The MP of Bi is usually low due to more tendency of Bi to form three bonds than 5 bonds (Inert pair effect). Thus there are weak forces of attraction between their atoms in solid state. Chemical Properties: Nature of Bonding: The group 15 elements can form bonds either by transfer of electrons or by sharing of electrons. a) By transfer of electrons: The valence shell electronic configuration of group 15 is n2np3.It shows that it is not so easy to lose all 5 electrons to form M+5 rather they can accept 3 electrons to form M –3. Electron accepting tendency of N is high due to its small size. P can form P –3. But electron accepting tendency decreases from N to Bi due to increase in atomic size from N to Bi. b) By sharing of electrons: In order to attain ns2np6 configuration the elements can share their valence p –electrons with electrons of other elements to form covalent bonds as NH3, PH3, PCl3, SbCl3 etc., They can also form homoatomic molecules such as N2, P4, As4, and Sb4 due to electron sharing. Maximum Covalency: General electronic configuration of group 15 is ns2np3 hence expected covalence is 3. Nitrogen shows maximum covalency of 4 in NH4Cl by donating electron pair on the nitrogen atom. But P and remaining elements can utilize their vacant d-atomic orbitals (due to the promotion of one electron from s-orbital) thereby show the covalency of 5 or six in their compounds PCl5,AsCl5, [PF6] –, [SbF6] –. In addition to this the d orbitals can be involved in the multiple bonding by sidewise overlapping to form dπ – pπ bonds in compounds of the type R3P = O and R3P = CH2. Both P and As can also form dπ – dπ bonds with some transition metals when they are in the form of P(C2H5)3 and As(C6H5)3 as ligands. Oxidation States: The valence shell electronic configuration of group 15 elements is ns2np3 .They can exhibit oxidation states of –3, +3 and +5 .The elements N and P exhibit –3 oxidation states in their compounds like Mg3N2, Ca3N2 etc .and Ca3P2, Mg3P2 due to their high electro negativities and small size with highly electropositive elements. However all the members of group –15 have tendency to exhibit variable oxidation states of +3 and +5. The stability of +3 increases and that of +5 decreases down the group due to inert pair effect. Inert pair effect: It represents the inertness of the valence s-electrons of heavier elements of p-block elements to take part in bond formation. This is due to ineffective screening effect of d and f-electrons. Inert pair effect is maximum in Bi due to large nuclear charge. Hence, BiCl3 can exist but not BiCl5. Oxidation states of Nitrogen: Nitrogen shows all oxidation states from –3 to +5 in its compounds as shown below. Compound Oxidation state of N Compound Oxidation state of N NH3 –3 N2O +1 N2H4 –2 NO +2 NH2OH –1 HNO2 +3 N2 0 NO2 +4 HNO3 +5 Chemistry p –BLOCK ELEMENTS Page 2 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 The compounds of nitrogen in +3 oxidation state tend to disproportionate in acid medium. Example: 3HNO2 ⟶ HNO3 + 2NO + H2O. Disproportionation: Nitrogen undergoes disproportionation in all its oxidation states in acidic medium. Example: 3HNO2 → HNO3 + 2NO + H2O Disproportionation is also observed in P as shown below. 4H3PO3 → 3H3PO4 + PH3 Remaining members of the family have lesser tendency to undergo disproportionation. Bi(V) is stronger oxidizing agent than Sb(V). Give reason. This is because Bi(V) is less stable than Sb(V), hence Bi(V) is readily reduced to Bi(III) which is more stable than Sb(III) on account of inert pair effect. Anomalous Properties of Nitrogen: Cause for the Anomalous behaviour of Nitrogen: a) Nitrogen has small size. b) It has high electronegativity. c) high ionisation enthalpy. d) It does not possess vacant d-orbitals in its valence shell. Anomalous Properties of Nitrogen: 1) It is least reactive due to high stability of N≡N in which two N-atoms are held together by triple bond hence, it has high bond strength. 2) The other elements of the group like P, As and Sb form tetrahedral molecules in their elemental state with the formula E4 but nitrogen does not form N4 molecule. 3. It has unique ability to form pπ- pπ multiple bonds with itself and other elements of small size and high electronegativity (O and C). Other elements like P,As and Sb form tetrahedral molecules in elemental state. 4. It is unable to form dπ- pπ bonds due to absence of d-orbitals and its covalancy is restricted to 4. The other elements of the group do not form pπ –pπ multiple bonds easily but multiple bonding of the type dπ-dπ can readily takes place in these elements. Chemical Properties: Reactivity towards H2: Group-15 elements form hydrides having general formula EH3.All are covalent in nature. Example: NH3(Ammonia), PH3(Phosphine), AsH3(Arsine), SbH3(Stibine) , BiH3(Bismuthine). Structure of Hydrides: In all hydrides the central atom undergoes sp3 hybriisation. Three of four hybrid orbitals form sigma bonds with H atom where as fourth hybrid orbital contains lone pair. Hence they have pyramidal structure. Properties of Hydrides of Group-15: 1. Bond angle: The H- E-H bond angle decreases from N to Sb. This is because, electronegativity of central atom decreases from N to Sb, hence electron density around the central atom also decreases consequently repulsion interaction between the pair also decreases. Basic strength: The Hydrides of group 15 are basic due to presence of long pair of electrons on central atom. Basic strength decreases in the order. NH3> PH3>AsH3>SbH3>BiH3. This is because, the atomic size increases from N to Bi thereby electron density around the central atom decreases. Hence, tendency to donate electron pairs decreases. Thermal stability: The thermal stability decreases NH3 to BiH3. It is inversely proportional to E-H bond length. The E-H bond length increases from NH3 to BiH3 due to increase in atomic size of the central atom from N to Bi. Reducing nature: Reducing nature of EH3 increases from NH3 to BiH3. This is because thermal stability decreases from NH3 to BiH3 hence ability to release H-atom increases from NH3 to BiH3, hence reducing nature increases from NH3 to BiH3. BiH3 is strongest reducing agent among hydrides of group 15. Reactivity towards oxygen: Group-15 form two types of oxides E2O3 and E2O5. An oxide in its higher oxidation state is more acidic than the oxide in its lower oxidation state. Hence, E2O5 is more acidic than E2O3. Acidic character of a given oxide decreases down the group. Chemistry p –BLOCK ELEMENTS Page 3 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 The oxides N2O3 and P2O3 are acidic, the oxides As2O3 and Sb2O3 are amphoteric and Bi2O3 is predominately basic. Nature of oxides: All oxides of nitrogen (except NO and N2O) and phosphorus are strongly acidic. Oxides of arsenic are weakly acidic. Oxides of antimony are amphoteric and those of bismuth are weakly basic. Reason: This can be explained on the basis of size of atoms. Nitrogen atom is small and interacts water more strongly pulling the electron pair between O –H and thus helps the release of H+ ions. However, this tendency decreases with increase in size and thereby acidic character decreases and conversely increases the basic character. Stability of oxides: The stability oxides of elements in their higher oxidation states decrease as we move down the group. Reactivity towards metals: Group 15 elements react with metals to form binary compounds exhibiting – 3 oxidation state. Example: Ca3N2 (calcium nitride), Ca3P2(Calcium phosphide), Na3As(Sodium arsenide), Zn3Sb2( Zinc antimonide) and Mg3Bi2(Magnesium bismuthide). DINITROGEN: Preparation of nitrogen: 1. Laboratory preparation: It is prepared by the action of NH4Cl solution on NaNO2 solution. NH4Cl + NaNO2 → N2 + 2H2O + NaCl Small amounts of NO and HNO3 are also formed in this reaction and are removed by passing the gas through aqueous sulphuric acid containing potassium dichromate. 2. Preparation from Ammonium dichromate: Nitrogen can be prepared by the thermal decomposition of ammonium dichromate. (NH4)2Cr2O7 → N2 + 4H2O + Cr2O3. 3. Preparation from Barium azide or sodium azide: When barium azide or sodium azide is heated thermal decomposition takes place to form N2 gas. Heat 573𝐾 Ba(N3)2 → Ba + 3N2 2 NaN3 → 2 Na + 3N2 Properties of Nitrogen: Physical properties: It is a colourless, oourless, tasteless and non toxic gas. It has two stable isotopes N 14 and N15. It has very low solubility in water (23.2cm3per litre of water at 273K and 1bar pressure) and low freezing and boiling points. It highly inert at room temperature due to high bond enthalpy of N ≡ N bond and is highly reactive at high temperatures. Chemical Properties: 1. Formation of Nitrides: It combines with some metals directly at high temperature to form ionic nitrides. Heat Heat 6Li + N2 → 2 Li3N 3Mg + N2 → Mg3N2 2. Action of H2: It combines with H2 at 773K to form ammonia. N2 + 3H2 ⇌ 2NH3 3. Action of O2: It combines with oxygen at 2000K to form nitric oxide. N2 + O2 ⇌ 2 NO Uses of nitrogen: 1. It is used in the manufacture of ammonia.2. It is used to create inert atmosphere. 3. Liquid nitrogen is used as refrigerant to preserve biological materials, food items and in cryosurgery. Compounds of Nitrogen: AMMONIA: Preparation of ammonia: 1. Ammonia in air and soil is formed by the decay of nitrogenous organic matter. Example: Urea NH2CONH2 + 2H2O ⟶ (NH4)2CO3 ⟶ 2NH3 + H2O + CO2 2. From ammonium salts: When ammonium salts are treated with lime or sodium hydroxide decomposition takes place to form NH3. 2NH4Cl + 2Ca(OH)2 ⟶ 2NH3 + 2H2O + CaCl2 (NH4)2SO4 + 2NaOH ⟶ 2NH3 + 2H2O + Na2SO4 Chemistry p –BLOCK ELEMENTS Page 4 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Ammonia is an important hydride of nitrogen. It is a weak base and forms ammonium salts when it reacts with acids.Haber’s process is principal method of fixing atmospheric nitrogen. Manufacture: Ammonia is manufactured by Haber’s process from N2 and H2. The reaction is reversible, exothermic and proceeds with the decrease in volume. N2(g) + 3H2(g) ⇌ 2NH3 (g) : H = -92.4 kJ --------- (1) According to Le-Chatelier’s principle the favourable conditions for the forward reactions are low temperature and high pressure. However, at low temperature the reaction is slow hence the following optimum conditions are maintained. i) Pressure of about 200atm ii)Optimum temperature of 4500C iii) Finely divided iron as catalyst supported by molybdenum or mixture of K2O and Al2O3 as promoter. The yield of ammonia is further increased by removing ammonia from equilibrium continuously. Structure of Ammonia:In NH3 the N atom undergoes sp3 hybridisation. It contains four sp3 hybrid orbitals. Among these three half filled sp3 hybrid orbitals overlap with half filled 1s atomic orbitals of three hydrogen atoms along the axis to form three N – H bonds. The fourth hybrid orbital of N contains lone pair of electrons. In NH3 regular tetrahedral configuration with 109281 is expected. But, it has distorted tetrahedral geometry (Pyramidal) due to lone pair-bond pair repulsion. Ammonia has relatively higher melting and boiling points on account of association due to hydrogen bonds. Properties of Ammonia: 1. Basic nature: The aqueous solution of ammonia is weak basic due to the formation of OH –. NH3(g) + H2O ⇌ NH4+ (aq) + OH –(aq). 2. Action of acids: It reacts with acids to form respective salt. NH3 + HCl → NH4Cl 2NH3 + H2SO4 → (NH4)2SO4 3. Action of Zinc sulphate : It reacts with ZnSO4 solution to form precipitate of zinc hydroxide. ZnSO4(aq) + 2NH4OH → Zn(OH)2 + (NH4)2SO4 4. Action of ferric chloride: Ammonia solution (NH4OH) reacts with ferric chloride solution to form brown ppt of hydrated ferric oxide. FeCl3(aq) + NH4OH (aq) ⟶ Fe2O3.xH2O(s) + NH4Cl 5. Formation of complexes: NH3 as a ligand forms complexes wit metal ions due to the presence of lone pair of electrons. i) It reacts with Cu+2 ions in solution to form cuprammoinium complex ion. Cu+2(aq) + 4NH3 →[Cu(NH3)4]+2. ii) It reacts with AgCl in solution to form diamminesilver(I)chloride complex . AgCl(s) (curdy white ppt) + 2NH3 ⟶ [Ag(NH3)2]Cl (aq) (colourless). Chemistry p –BLOCK ELEMENTS Page 5 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Uses of ammonia: Ammonia is used 1. in the manufacture of nitric acid. 2. in the manufacture of Na2CO3 by Solvay’s process 3. in the manufacture of nitrogenous fertilizers like ammonium nitrate, ammonium sulphate, calcium ammonium nitrate(CAN)etc. 4. as a laboratory reagent 5. to prepare cuprammonium sulphate solvent for cotton in the manufacture of rayon. 6. liquid ammonia is used as refrigerant in ice factory and cold storages. 7. A mixture of ammonium nitrate and aluminium powder is used explosive under the name Amonal. Similarly mixture of ammonium nitrate and TNT is used as an explosive called Amatol. 8. As cleaning agent for removing grease in dry cleaning. 9. to prepare sal ammoniac (NH4Cl)used in dyeing industry, in textile printing, and in dry cells. NOTE: Bottles containing liquor ammonia should be cooled before opening. Why? Reason: The vapour pressure of ammonia is high. NITRIC ACID: Nitrogen forms oxyacids such as H2N2O2 (Hyponitrous acid), HNO2 and HNO3. Earlier it was called ‘aqua fortis meaning ‘strong water’ as it attacks almost all metals. Preparation: It is prepared by heating sodium nitrate with conc. H2SO4 in glass retort. NaNO3 + H2SO4 → NaHSO4 + HNO3 Manufacture of Nitric acid: Ostwald’s process: The following steps are involved in the manufacture of nitric acid. 1. Ammonia from Haber’s process is oxidised to NO using atmospheric oxygen at 500K in presence of platinum/Rhodium guaze catalyst under 9 bar pressure. 4NH3 + 5O2 → 4 NO + 6H2O. 2. NO formed is oxidised to NO2 in presence of oxygen. 2NO + O2 ⇌ 2 NO2 3. It dissolves in water to form nitric acid. 3NO2 + H2O → 2HNO3 + NO The nitric acid formed is concentrated by distillation up to 68%. It is further concentrated up to 98% by dehydration using conc. H2SO4. Properties of nitric acid: 1. Anhydrous nitric acid is colourless, fuming and pungent smelling liquid. It decomposes slowly in presence of sunlight and acquires yellow colour. 2. It boils at 355.6K and freezes at 231.4K. 3. 68% nitric acid forms azeotropic mixture with water which boils at 394K. Structure of nitric acid: O H O N M O Chemical properties: 1. Acidic nature: In aqueous solution HNO3 acts as a strong acid giving hydronium ion and nitrate ion. HNO3 + H2O → H3O+ + NO3– 2. Action on metals: It oxidises most of metals to form respective metal nitrates. a) Concentrated nitric acid reacts with Cu to form copper nitrate and NO2 gas is liberated. Cu + 4HNO3 → Cu(NO3)2 + 2NO2 + 2H2O b) Dilute nitric acid reacts with Cu to form copper nitrate and NO gas is liberated. 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O c) Concentrated nitric acid reacts with Zn to form zinc nitrate and NO2 gas is liberated. Zn + 4HNO3 → Zn(NO3)2 + 2NO2 + 2H2O d) Dilute nitric acid reacts with Zn to form zinc nitrate and N2O gas is liberated 4Zn + 10HNO3 → 4Zn(NO3)2 + N2O + 5H2O Passivity: When metals like Cr, Ni, Fe, and Al are dipped in concentrated nitric acid they lose their normal reactivity and become passive. This is due to the formation of a thin protective layer of the metal oxide on the surface of the metal which prevents further action. Chemistry p –BLOCK ELEMENTS Page 6 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE Action on non-metals: Concentrated nitric acid oxidises non metals to their respective oxy-acids. a) Conc.HNO3 oxidises C to carbonic acid. C + 4HNO3 → H2CO3 + 4NO2 + H2O b) Conc.HNO3 oxidises I2 to iodic acid. I2 + 10HNO3 → 2HIO3 + 10NO2 + 4H2O c) Conc.HNO3 oxidises S8 to sulphuric acid. S8 + 48HNO3 ⟶ 8H2SO4 + 48NO2 + 16H2O d) Conc.HNO3 oxidises P4 to phosphoric acid. P4 + 20HNO3 ⟶ 4H3PO4 + 20NO2 + 4H2O Brown Ring Test: The presence of nitrate ion in a salt can be detected by Brown ring test. An aqueous solution of the salt is mixed with equal volume of freshly prepared ferrous sulphate solution. To this conc. sulphuric acid is added along the sides of the test tube till a dark brown ring appears. This indicates the presence of NO3– ion in a given salt. NO3 – + 3Fe+2 + 4H+ → NO(g) + 3Fe+3 + 2H2O Fe+2 + NO + 5H2O → [Fe(H2O)5NO]+2 Uses of Nitric acid: 1.It is used in the manufacture of explosives like TNT, Dynamite, Picric acid etc., 2. It used in the manufacture of fertilizers like ammonium nitrate and basic calcium nitrate[CaO.Ca(NO3)2]. 3. It is used in the manufacture of artificial silk. 4. It is used in the purification of gold and platinum as aqua regia. 5. It is used in the preparation of nitro compounds which are used in perfumes, dyes, medicine etc., STRUCTURE OF SOME OXIDES OF NITROGEN: Nitrogen (I) oxide or Dinitrogen oxide: Preparation: It is prepared by decomposing ammonium nitrate. Heat NH4NO3 → It is a colourless neutral gas. Structure: N2O + 2H2O Nitrogen monoxide orNitrogen(II)oxide: Preparation: It is prepared by the action of acidified ferrous sulphate on sodium nitrite. 2NaNO2 + 2FeSO4 + 3H2SO4 ⟶ Fe2(SO4)3+ 2NaHSO4 + 2H2O + 2NO It is a colourless neutral gas. Structure: NO has 11 electrons ( 5 due to N and 6 due to O ). This means that one electron is unpaired hence, it is paramagnetic. Dinitrogen trioxide or Nitrogen(III)oxide: Preparation: It is prepared by the action of nitrogen monoxide on dinitrogen tetroxide at 250K: 250K 2NO + N2O4 → 2N2O3 It is bluish colored solid and is acidic in nature. Structure: Nitrogen dioxide or Nitrogen(IV)oxide: It is a brown coloured gas and is acidic. Preparation: It is prepared by the decomposition of lead nitrate at 673K. 673K 2Pb(NO3)2 → Chemistry 4NO2 + 2PbO + O2 p –BLOCK ELEMENTS Page 7 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE It is a brown coloured gas and is acidic in nature. Structure: NO2 molecule has 17 electrons hence, it has unpaired electron. It is a odd electron molecule and is paramagnetic. It is a mixed anhydride as it reacts with water to give HNO2 and HNO3. 2NO2 + H2O → HNO2 + HNO3 Dinitrogen tetroxide or Nitrogen(IV)oxide: Preparation: N2O4 is in equilibrium with NO2, hence when brown coloured NO2 is cooled it will be conveted into colourless N2O4. 2NO2 ⇌ N2O4. It colourless liquid/solid and is acidic in nature. Structure: Nitrogen Pentoxide or Nitrogen(V)oxide: Preparation: It is prepared by the action of nitric acid on phosphorus pentoxide. 4HNO3 + P4O10 ⟶ 4HPO3 + 2N2O5 It is colourless solid. It is anhydride of HNO3 and is acidic. Structure: PHOSPHORUS: Occurance: It is a reactive non-metal and does not exist in free state. The important minerals of phosphorus are 1. Phosphorite: Ca3(PO4)22. Fluorapatite: 3Ca3(PO4)2.CaF2. 3. Chlorapatite: 3Ca3(PO4)2.CaCl2 4. Hydroxyapatite: 3Ca3(PO4)2.Ca(OH)2. Phosphorus constitutes our bones, teeth, muscles and nerve tissues. It is also present in milk, eggs, fish and beans in the form of phospholipids. Allotropic forms of Phosphorus: Phosphorus exists in three main allotropic forms a) White phosphorus b) Red phosphorus c) Black phosphorus. White Phosphorus: It is a translucent white waxy solid. White phosphorus is insoluble in water but soluble in carbon disulphide. It glows in dark (Chemiluminescence). It has garlic odour. It dissolves in boiling NaOH in an inert atmosphere to form PH3. P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2(sodium hypophosphite) Upon exposure to air it becomes yellow, hence it is also called yellow phosphorus. Structure: White phosphorus exists as P4 molecule in which each P atom is linked to three other atoms by covalent bonds and has a lone pair of electrons on it. P – P – P bond angle is 600 and the molecule is under strain. Hence, it is highly reactive. P P P P Chemistry p –BLOCK ELEMENTS Page 8 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE White phosphorus Action of air: White phosphorus readily catches fire in air to give dense white fumes of P4O10. P4 + 5O2 → P4O10 Red phosphorus: Preparation: When white phosphorus is heated in an inert atmosphere of CO2 or coal at 573K for several days red phosphorus is obtained. White P4 → Red P4. Red P4 also consists of P4 molecules but these are linked by covalent bonds resulting in polymeric structure. P P P P P P P P P P P P P P P P Red phosphorus Properties: 1) Red phosphorus is odourless powder and is non-poisinous. 2. It is insoluble in water and carbon disulphide but soluble in alcohol and ether. 3. It is less reactive than white phosphorus. 4. Its ignition temperature (543K) is more than that of white phosphorus(303K). 5. It sublimes on heating. When vapours are condensed white phosphorus is obtained. This is due to the cleavage of polymeric structure. Black Phosphorus: It is the most inactive form phosphorus as compared to red and white phosphorus. It has polymeric structure consists of double layered crystal lattice in which each layer is made up of zigzag chains linking phosphorus atoms. It exists in two forms 1. α-Black phosphorus 2. β-Black phosphorus. Preparation of α-Black phosphorus: When Red phosphorus is heated in a sealed tube at 803K α-Black phosphorus is obtained. It has opaque monoclinic or rhombohedral crystals. It does not oxidise in air. Preparation of β-Black phosphorus: When white phosphorus is heated at 473K under high pressure βBlack phosphorus is obtained. It does not burn in air up to 673K. COMPOUNDS OF PHOSPHORUS: PHOSPHINE: (PH3): Preparation: 1. It can be prepared by the action of water or dil. HCl on calcium phosphide. Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3 Ca3P2 + 6HCl → 3CaCl2 + 2PH3 2. Laboratory preparation: When white P4is heated with conc. NaOH in an inert atmosphere of CO2 phosphine is obtained. P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2(sodium hypophosphite) In addition to PH3 phosphorus dihydride (P2H4) is formed which is highly inflammable. 3P4 + 8NaOH + 8H2O → 8NaH2PO2 + 2P2H4. As soon as P2H4 is formed gas come in contact with air it catches fire spontaneously forming smoke ring called VORTEX RINGS. 2P2H4 + 7O2 → 4HPO3(meta phosphoric acid) + 2H2O However PH3 is not spontaneously inflammable. It burns at 473K. 2PH3 + 4O2 → P2O5 + 3H2O. Purification: PH3 is purified by absorbing it by HI to form phosphonium iodide (PH4I). PH4I is treated with KOH to form pure PH3. PH4I + KOH → KI + H2O + PH3. Properties of PH3: Physical properties: Colourless gas with rotten fish smell. It is highly poisonous. It explodes in presence of oxidizing agents like HNO3, Cl2, and Br2 vapours. Chemistry p –BLOCK ELEMENTS Page 9 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE Chemical properties: Action of water: It is slightly soluble in water. The solution of PH3 in water decomposes in presence of light giving red phosphorus and hydrogen. Action of CuSO4: It reacts with CuSO4 solution to give cupric phosphide. 3CuSO4 + 2PH3 ⟶ Cu3P2 + 3H2SO4 Action of HgCl2: It reacts with HgCl2 solution to give mercuric phosphide. 3HgCl2 + 2PH3 ⟶ Hg3P2 + 6HCl Basic nature: It is a weak base. It reacts with acids to form phosphonium compounds. PH3 + HBr → PH4Br Structure of PH3 and PH4+: Uses of PH3: 1. It is used to prepare smoke screens in war fare. 2. It is used in Holme’s signals. A mixture of CaC2 and Ca3P2 is taken in container with a hole in it. It is immersed in water near the rock. As soon as water enters the hole both acetylene and PH3 are formed.PH3 catches fire in air and lights up acetylene. This act as signal for the approaching ship. Phosphorus trichloride: Preparation of phosphorus trihalide: 1. When dry chlorine is passed over heated white phosphorus to form phosphorus trichloride. P4 + 6Cl2 ⟶ 4PCl3 2. It is prepared by the action of thionyl chloride on white phosphorus. P4 + 8SOCl2 ⟶ 4PCl3 + 4SO2 + 2S2Cl2 Properties: It is a colourless oily liquid. Action of water: In the presence of moisture it undergoes hydrolysis to form phosphorus acid. PCl3 + 3H2O ⟶ H3PO3 + 3HCl Action on Acetic acid and ethyl alcohol: It reacts with organic compounds containing –OH and replaces –OH by –Cl to form chloro derivatives as shown below. 3CH3COOH + PCl3 ⟶ 3CH3COCl + H3PO3 3C2H5OH + PCl3 ⟶ 3C2H5Cl + H3PO3 Structure of PCl3: The geometry of PCl3 is pyramidal shape phosphorus undergoes sp3 hybridisation Phosphorus Pentachloride (PCl5): Preparation: 1. White phosphorus reacts with excess of dry chlorine to form phosphorus pentachloride P4 + 10Cl2 ⟶ 4PCl5 2. It is prepared by the action of SO2Cl2 on phosphorus. P4 + 10SO2Cl2 ⟶ 4PCl5 + 10 SO2 Properties: 1. It is a yellowish white powder. 2. Action of water: It undergoes hydrolysis in the presence of moist air to form phosphoric acid. PCl5 + 4H2O ⟶ H3PO4 + 5HCl 3. Action of heat: It sublimes on heating and decomposes on strong heating to form PCl3. Heat PCl5 → Chemistry PCl3 + Cl2 p –BLOCK ELEMENTS Page 10 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE 4. Action on organic compounds: It reacts with organic compounds containing –OH and replaces –OH by –Cl to form chloro derivatives as shown below. CH3COOH + PCl5 ⟶ CH3COCl + POCl3 + HCl C2H5OH + PCl5 ⟶ C2H5Cl + POCl3 + HCl 5. Action of metals: PCl5 reacts with finely devided metals on heating to form corresponding chlorides. 2Ag + PCl5 ⟶ 2AgCl + PCl3 Sn + 2PCl5 ⟶ SnCl4 + 2PCl3 Structure of PCl5: In gaseous and liquid states PCl5 has trigonal bipyramidal structure. The three equatorial bonds are equivalent while two axial bonds are longer than equatorial bonds. This is due to bond pair- bond pair repulsion between axial and equatorial bonds.In solid state it exists as ionic solid[PCl4]+[PCl6]– Cation[PCl4]+ is tetrahedral and anoin [PCl6]– is octahedral. Oxoacids of Phosphorus: Some of the oxoacids of phosphorus are listed as follows. SL Name of Oxoacid Formula O.N. of Basicity Reducing Property No. Phosphorus Hypophosphorus acid. Reducing with two 1 H3PO2 +1 1 P –H linkages. Orthophosphorus acid Reducing with one 2 H3PO3 +3 2 P – H linkage. Orthophosphoric acid Non-reducing with no 3 H3PO4 +5 3 P – H linkage Pyrophosphorus acid Reducing with two P –H 4 H4P2O5 +3 2 bonds Hypophosphoric acid Non-reducing with no 5 H4P2O6 +4 4 P – H linkage Pyrophosphoric acid Non-reducing with no 6 H4P2O7 +5 4 P – H linkage Metaphosphoric acid Non-reducing with no 7 (HPO3)3 +5 3 P – H linkage Structure of Some Oxoacids of Phosphorus: Chemistry p –BLOCK ELEMENTS Page 11 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE Characteristics of Ox oacids of Phosphorus: 1. In all oxo acids phosphorus is tetrahedrally surrounded by four other atoms or groups. 2. All oxo acids contain one P = O and at least one P –OH group. 3. The oxo acids in which phosphorus has oxidation state less than +5 contain either P –H or P –P bonds but not both in addition to P = O and P –OH bonds. 4. The oxo acids in +3 oxidation state undergo disproportion reaction to yield compounds in higher and lower oxidation state. Heat Example: 4 H3PO3 → PH3 (ON of P = –3) + 3H3PO4 (ON of P = +5) 5. Acids which contain P –H bonds have strong reducing properties. Example: Hypophosphorus acid (H3PO2) contains two P –H bonds and reduces AgNO3 to metallic silver and HgCl2 to Hg. 4AgNO3 + H3PO2 + 2 H2O ⟶ 4Ag + H3PO4 + 4HNO3 2HgCl2 + H3PO2 + 2 H2O ⟶ 2Hg + H3PO4 + 4HCl H3PO3 weaker reducing agent than H3PO2 as it contains only one P –H bond as compared to two P –H bonds in H3PO2. 6. P – H bonds are not ionisable to give H+ and do not play any role in basicity. Only those H atoms which are attached with oxygen in P – OH form are ionisable and cause basicity. Example: H3PO3 – Dibasic (Two P – OH bonds). H3PO4 – tribasic (Three P – OH bonds) GROUP-16 ELEMENTS: Introduction: Oxygen, sulphur, selenium, tellurium and polonium belongs to group 16 of the periodic table. These are also called as chalcogens (ore forming) because most of the metals occur in nature as oxides or sulphides. Occurrence: Oxygen is the most abundant element on the earth crust. Sulphur occurs on the earth crust to an extent of 0.05%. Sulphur exists in the form of combined state also. Ex: CaSO 4 . 2H2O gypsum MgSO4 . 7H2O Epsom, BaSO4 Baryte, PbS – Galena, ZnS – Zinc blende, CuFeS2 copper pyrites. Selenium and Tellurium are found as metal selenides and tellurides. Polonium is a radioactive element. It is formed by the radioactive disintegration of Thorium and Uranium minerals. Electronic Configuration The general electronic configuration of group 16 elements is [noble gas] ns2 np4. Si.No. Element Symbol Atomic number Electronic Configuration 1 Oxygen O 8 [He]2s22p4 2 Sulphur S 16 [Ne]3s23p4 3 Selenium Se 34 [Ar]3d104s24p4 4 Tellurium Te 52 [Kr]4d105s25p4 5 Polonium Po 84 [Xe]4f14 5d106s26p4 2 4 Oxidation states: All the elements of group 16 have ns np electronic configuration in their outermost shell. They tend to acquire the noble gas configuration either by sharing of electrons or by gaining electrons. Oxygen exhibits –2 oxidation state by gaining two electrons due to its high electronegativity. In addition to this oxygen also exhibits oxidation state of –1 in peroxides, –1/2 in superoxides, zero in O2 Chemistry p –BLOCK ELEMENTS Page 12 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 and O3, +2 in OF2, and +1 in O2F2.The tendency to exhibit –2 oxidation state decreases down the group due to the decrease in electronegativity from oxygen to Polonium. The other elements have tendency to exhibit +2, +4 and +6 oxidation states. This is due to the availability of vacant d-orbitals in these elements except oxygen. In the ground state these elements have two unpaired electrons and hence, can form two bonds. This explains their +2 oxidation state. In the first excited state one of the paired p-electron goes to the vacant d-orbital of the same shell, thus making four unpaired electrons available for bonding. This accounts for their +4 oxidation state. On further excitation one of the paired s-electrons also gets promoted to d-orbital that makes availability of six unpaired electrons. This explains +6 oxidation state. The stability of +6 oxidation state decreases down the group due to inert pair effect. Thus +6 is most stable in case of S and least stable in case of Po. In general the compounds of S, Se, Te and Po with oxygen are tetravalent. They show both oxidising and reducing properties. The elements S, Se, Te and Po form compounds in +6 state with highly electronegative fluorine. In +6 state they act as oxidising agents. Atomic and Ionic Radii: The atomic and ionic radii increases down the group (from oxygen to polonium) due to the addition of one new shell at each element down the group. Ionisation Enthalpy: Ionisation energy decreases down the group due to the increase in the atomic size. 16 group elements have lower ionization enthalpy compare to 15 group elements. Because the 15 group elements have extra stable half filled p-orbital configuration. This gives extra stability to the 15 group elements. Electron gain enthalpy: Electron gain enthalpy decreases (becomes less negative) down the group. This is due to the increase in the atomic radii. The electron gain enthalpy of oxygen is less negative than that of S due to very small size, its electron charge density is high. Due to this incoming electron experiences more repulsion from electron on the oxygen atom. Hence Oxygen has least tendency to accept an outside electron as compared to that of S. Sulphur has highly negative electron gain enthalpy due to bigger size and lesser crowding of electron around it. Electronegativity: Electronegativity decreases with an increase in the atomic number. (Decreases from O to Po). This is due to the increase in the atomic radii and the metallic nature of the elements increases from oxygen to polonium. Oxygen is highly electronegative element. Melting and Boiling points: The melting and boiling points increases down the group because the strength of Vander Waal’s forces increases. Oxygen has low melting and boiling points than sulphur because oxygen exists as a diatomic molecules and the sulphur exists as a polyatomic molecule having a ring structure. The atoms in the rings are held by covalent bonds. The M.P. and B.P. of Po are lower than Te, due to inert-pair effect. The s-electron pair in polonium is not easily available so the Vander Waal’s forces become weaker. Reactivity with hydrogen: All the elements of group 16 form H2E type of hydrides (E=O,S, Se, Te, Po).The acidic character increases from H2O to H2Te due to decrease in the bond enthalpy for the dissociation of H – E bond down the group. Thermal stability of hydrides decreases form H2O to H2Po due to decrease in the bond enthalpy for the dissociation of H – E bond down the group. Except H2O the remaining hydrides acts as a reducing agents. The reducing power increases from H2S to H2Te. Reactivity with oxygen: Group 16 elements form EO2 and EO3 type of oxides.(E=S,Se,Te and Po). Reducing properties decreases from SO2 to TeO2. SO2 is a reducing agent and TeO2 is an oxidizing agent. Both the oxides are acidic in nature. Since, +6 oxidation state of S is more stable than +4, SO2 acts as a reducing agent. Since, the stability of +6 oxidation state decreases from S to Te the reducing character of dioxides decreases while their oxidising character increases. Thus TeO2 acts as an oxidising agent. The EO3 type oxides like SO3, SeO3 and TeO3 are acidic in nature. Chemistry p –BLOCK ELEMENTS Page 13 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Reactivity with Halogens: Group 16 form halides of the type EX6, EX4 and EX2 type where E is group 16 element and X is halogen. The stability of halides decreases in the order F – > Cl – > Br – > I –. Hexahalides: Amongst hexahalides hexafluorides are the only stable halides. All hexafluorides are gaseous in nature with octahedral structure. SF6 is exceptionally stable stable for steric reasons. Tetrahahalides: Amongst tetrafluorides SF4 is a gas, SeF4 is a liquid and TeF4 a solid with sp3d hybridization having trigonal bipyramidal structures in which one of the equatorial positions is occupied by a lone pair of electrons. Hence geometry is see-saw geometry. Dihalides: Except selenium all elements form dichlorides and dibromides with sp3 hybridisation having tetrahedral structure. Monohalides: The monohalides are dimeric in nature like S2F2, S2Cl2 S2Br2, Se2Cl2 and Se2Br2. The dimeric monohalides undergo disproportionation as follows. 2Se2Cl2 ⟶ SeCl4 + 3Se Anamalous behaviour of oxygen: 1) Oxygen at room temperature is a gas, the remaining elements are solids. 2) Oxygen molecule exists as a diatomic, the remaining elements are polyatomic in nature. 3) Oxygen compounds are more ionic in nature compare to the compounds of other elements. 4) Oxygen forms strong hydrogen bonds, the other elements of group 16 do not form hydrogen bonds. 5) Oxygen is paramagnetic, the remaining elements are diamagnetic. Cause for the Anomalous behaviour of oxygen 1) Oxygen atom is small in size. 2) Electronegativity of oxygen is high. 3) Non-availability of vacant d-orbitals in oxygen. DIOXYGEN: Preparation: 1. From potassium chlorate KClO3: When potassium chlorate heated in presence of MnO2 catalyst decomposition takes place to form O2. MnO2 2KClO3 → 2KCl + 3O2 2. By decomposition of heavy metal oxides: The oxides of heavy metals which are present low in the electrochemical series undergo decomposition to form dioxygen. 2Ag2O(s) ⟶ 4Ag(s) + O2(g) 2HgO(s) ⟶ 2Hg(l) + O2 2Pb3O4(s) ⟶ 6PbO(s) + O2(g) 2PbO2 (s) ⟶ 2PbO(s) + O2(g) 3. By decomposition of hydrogen peroxide: Hydrogen peroxide readily decomposes in presence of finely divided metals and manganese oxide catalysts to form water and dioxygen. 2H2O2(l) ⟶ 2H2O(l) + O2(g). 4. By electrolysis of water: On large scale dioxygen can be prepared by electrolysis of water. On electrolysis of water hydrogen is liberated at cathode and dioxygen at anode. 5. Industrially dioxygen is obtained from air by first removing CO2 and water vapour and then remaining gases are liquefied and fractionally distilled to give dinitrogen and dioxygen. Properties: Dioxygen is a colourless, odourless gas. It is soluble in water. Oxygen has three stable isotopes. 16O, 17O and 18O. Oxygen is paramagnetic in nature. It liquefies at 90K and freezes at 55K. Action of metals: When metals except Au and Pt react with dioxygen at high temperature to form corresponding metal oxides. The exothermic nature of reaction helps to sustain the reaction. The external heating is required to overcome high bond dissociation enthalpy. (493.4kJ/mol.)\ 4Al + 3O2 ⟶2Al2O3 2Ca + O2 ⟶ 2CaO Action of non-metals: Non metals like P4, and C react with oxygen to form their respective oxides. P4 + 5O2 ⟶ P4O10 C + O2 ⟶ CO2 Chemistry p –BLOCK ELEMENTS Page 14 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Action of CH4: Methane burns in presence of excess oxygen to form carbon dioxide and water. CH4(g) + 2O2(g) ⟶ CO2 + 2H2O(g) Action of ZnS: ZnS is oxidised to ZnO in presence of dioxygen. 2ZnS + 3O2 ⟶ 2ZnO + 2SO2 Catalytic oxidation: The compounds like SO2 and HCl catalytically oxidised to SO3 and Cl2 V2 O5 CuCl2 respectively. 2SO2 + O2 → 2SO3 4HCl + O2 → 2Cl2 + 2H2O. Uses of oxygen: 1) Oxygen is used in steel making. 2) Oxygen is used as an oxidizer in rocket propellants and in fuel cells. 3) Oxygen is used in oxy-hydrogen and oxy-acetylene welding torches. 4) Oxygen cylinders are widely used in hospitals. Simple oxides: A binary compound of oxygen with another element is called oxide. Simple oxides are classified on the basis of their acidic, basic and amphoteric nature. Acidic oxides: An oxide combines with water to give an acid is called acidic oxide. These are generally formed by combination of non-metals and oxygen. Example: P4O10, SO3, CO2, N2O5, B2O3 etc., SO2 + H2O H2SO3 (Sulphurous acid) SO3 + H2O H2SO4 (Sulphuric acid) CO2 + H2O H2CO3 (Carbonic acid) Basic oxides: An oxide combines with water to give a base is called basic oxide. These are generally formed by the combination of oxygen with highly electropositive metals. Example: MgO, K2O, CaO, BaO, Na2O. CaO + H2O Cu(OH)2(Calcium hydroxide) Amphoteric oxides: Some metallic oxides reacts with both acid and base are called amphoteric oxides. These are generally formed by the elements which are on borderline between the metals and non-metals. Example: Al2O3, SiO2, ZnO, PbO etc., Al2O3 + 6HCl + 9H2O 2[Al(H2O)6]+3 + 6Cl – Al2O3 + 6NaOH + 3H2O 2Na3[Al(OH)6] Some oxides neither acidic nor basic are called neutral oxides. Example: CO, NO and N2O. OZONE: Elemental oxygen exists in two molecular allotropic forms, O2 and O3. The triatomic molecule O3 is called trioxygen or ozone. Ozone is the allotropic form of oxygen. At a height of about 20km it is formed from atmospheric oxygen in presence of sun light. Preparation: When an electric discharge is passed through dry oxygen, ozone is formed. 𝑒𝑙𝑒𝑐𝑡𝑟𝑖𝑐 3O2 2O3(g) rH = 285 KJ 𝑑𝑖𝑠𝑐ℎ𝑎𝑟𝑔𝑒 Properties: 1.Pure ozone is a pale blue gas, dark blue liquid and violet black solid. 2. Ozone is thermodynamically unstable relative to oxygen 2O3(g) 3O2(g) rH = -284.5 KJ because decomposition of ozone into oxygen is an exothermic reaction, i.e., rH is negative and involves an increase in entropy i.e., rS is positive and change in Gibbs energy is also negative. 3. Ozone acts as a powerful oxidizing agent due to the reaction. O3 O2 + [O] During the decomposition of ozone nascent oxygen is formed. This nascent oxygen is responsible for the oxidation of number of substances. Action of PbS: Ozone oxidizes lead sulphide to lead sulphate. PbS(S) + 4O3(g) PbSO4(S) + 4O2(g) Action of NO: Nitrogen oxides emitted from the exhaust systems of supersonic jet aeroplanes slowly depleting the concentration of the ozone layer in the upper atmosphere. NO(g) + O3(g) NO2(g) + O2(g) Action of Iodide: It oxidises iodides into iodine. 2I – + H2O + O3 ⟶ 2OH – + I2 + O2 Chemistry p –BLOCK ELEMENTS Page 15 TARALABALU SCIENCE ACADEMY SIRIGERE 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Estimation of Ozone: Ozone is estimated by reacting ozone with excess of potassium iodide solution in presence of borate buffer (pH = 9.2)Iodine liberated is titrated against standard solution of sodium thiosulphate. Thereby amount of ozone can be estimated. Structure of Ozone: The ozone molecule bent structure with a bond angle of 1170. The two O –O bond length is identical with 128pm. It resonance hybrid of two main structures as shown below. Uses: 1. It is used as a germicide, disinfectant and for sterilizing water. 2. It is used in bleaching oils, starch, flour, etc. 3. It is used in the preservation of meat. 4. Ozone protects the earth’s surface from an harmful U.V. radiations. SULPHUR: Allotropic forms: Allotropic forms of sulphur are 1) Yellow rhombic sulphur (-sulphur). 2) Monoclinic () sulphur. Rhombic Sulphur:(𝛂-Sulphur) : 1) It is yellow in colour 2) Rhombic sulphur is prepared by dissolving powdered sulphur in carbon disulphide at room temperature. The mixture is then filtered. The CS2 will slowly evaporate leaving behind large octahedral crystals of rhombic sulphur. 3) Rhombic sulphur is insoluble in water but it is soluble in CS2. 4) M.P. is 385.8K and specific gravity is 2.06 g/ml. Monoclinic sulphur: (𝛃-Sulphur): It is stable only above 369K and transforms into α-Sulphur below 369K. At 369K both the forms ( and ) are stable and the temperature (369K) is called transition temperature. Monoclinic sulphur is prepared by melting rhombic sulphur in a dish and cooling, till crust is formed. Two holes are made in the crust and the remaining liquid poured out. On removing the crust, colourless needle shaped crystals of -sulphur are formed. Rhombic sulphur Chair form of Cyclo-S6. Above 1000 K sulphur is in the form of S2 and S2 is paramagnetic in nature. Which form of sulphur shows paramagnetic behavior? Why? Ans: In vapour state, sulphur exists as S2 molecule. It has two unpaired electrons in the antibonding orbitals. So, it shows paramagnetic nature. Sulphur Dioxide: Preparation: 1. From SO3 –2 salts: In laboratory when sulphite is treated with dil. H2SO4 gives sulphur dioxide. SO-23(aq) + 2H+(aq) H2O(l) + SO2(g) 2. From Sulphur: When sulphur is burnt in air or oxygen SO2 is formed with 6-8% SO3. S(s) + O2(g) ⟶ SO2(g) 3. From Iron pyrites: Industrially SO2 is produced as a byproduct of roasting of sulphide ores. 4FeS2 + 11O2 ⟶ 2Fe2O3 + 8SO2 Properties: Chemistry p –BLOCK ELEMENTS Page 16 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE Action of water: SO2 is a colourless gas with pungent smell and it is soluble in water. SO2(g) + H2O(l) H2SO3(aq) Sulphurous acid Action of NaOH: SO2 reacts with sodium hydroxide gives sodium sulphite, this again reacts with SO2 to form sodiumhydrogen sulphite 2NaOH + SO2 Na2SO3 + H2O Na2SO3 + H2O + SO2 2NaHSO3 Action of Cl2: SO2 reacts with chlorine in the presence of charcoal to give sulphuryl chloride (SO2Cl2) SO2(g) + Cl2(g) SO2Cl2(g) Reducing property of SO2: Action of Fe+3 salts: Moist SO2 behaves as a reducing agent. It converts Fe(III) to Fe(II) ions. 2Fe+3 + SO2 + 2H2O 2Fe+2 + SO4-2 + 4H+ Action of MnO4 – : Moist SO2 decolourises the acidified potassium permanganate solution. It is a test for SO2 gas. 5SO2 + 2MnO4 + 2H2O 5SO42 + 4H+ + 2Mn+2 Structure of SO2: Uses: SO2 is used 1) In refining petroleum and sugar. 2) In bleaching silk and wool. 3) As a disinfectant and preservative. 4) In the preparation of sodium hydrogen sulphite, calcium hydrogen sulphite, etc. Oxoacids of sulphur: Sulphur forms many oxoacids, structure of some oxoacids. Sulphuric acid: Manufacture by CONTACT process: Sulphuric acid is manufactured by contact process it involves three steps. 1. Burning of sulphur or sulphide ores in air to form SO2. SO2 is purified by removing dust and other impurities such as arsenic compounds. S(s) + O2(g) ⟶ SO2(g) OR 4FeS2 + 11O2 ⟶ 2Fe2O3 + 8SO2 2. Conversion of SO2 to SO3 by the reaction with oxygen in the presence of V2O5 catalyst. 𝑉2 𝑂5 2SO2(g) + O2(g) → 2SO3(g) rH = -196.6 KJ mol-1 The reaction is an exothermic, reversible and forward reaction leads to decrease in volume. Therefore low temperature and high pressure are the favourable conditions for maximum yield. (2 bar pressures 720K temp). 3. SO3 from the catalytic converter is absorbed in conc. H2SO4 to form oleum. When oleum is diluted with water gives H2SO4. This sulphuric acid is 96 – 98% pure. SO3 + H2SO4 H2S2O7 (Oleum) H2S2O7 + H2O 2H2SO4 (Sulphuric acid) Chemistry p –BLOCK ELEMENTS Page 17 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Flow chart for manufacture of Sulphuric acid by Contact process: 𝐀𝐢𝐫 → 𝐎𝐥𝐞𝐮𝐦 ← Sulphur Burner → Dust Precipitator →Washing and Cooling Drying Tower → Tower Absorption ← ← Pre ←Arsenic Catalytic Tower Converter Heater Purifier Properties: 1. H2SO4 is a colourless, dense, oily liquid. Acidic nature: H2SO4 dissolves in water and ionizes in two steps as shown below. H2SO4 + H2O H3O+ + HSO4 Ka1 = very large (> 10) + -2 HSO4 + H2O H3O + SO4 Ka2 = 1.2 x 10-2 The larger value of Ka1 means. H2SO4 is largely dissociated into H+ and HSO4 ions. Ka1 value is large means H2SO4 is a strong acid. Thus H2SO4 acts as a strong dibasic acid forms two series of salts –normal sulphates and acid sulphates or hydrogen sulphates or bisulphates Action of metal halides: H2SO4 is a low volatile liquid. So it can be used in the manufacture of more volatile acids from their salts. 2MX + H2SO4 ⟶ 2HX + M2SO4 [X = F, Cl and NO3] a) Action of KCl: When crystals of KCl reacts with conc. Sulphuric acid colourless fumes of HCl is formed. 2KCl + H2SO4 2HCl + K2SO4 b) Action of CaF2: When calcium fluoride reacts with hot conc.H2SO4 hydrogen fluoride is formed. CaF2 + H2SO4 ⟶ CaSO4 + 2HF Dehydrating property:Conc. H2SO4 has a very strong affinity for water. It removes hydrogen and oxygen from compounds in the form of water. Therefore it acts as a good dehydrating agent. Its corrosive action on skin is due to dehydration of the skin, followed by burning of the skin. It forms number of hydrates. H2SO4 + H2O H2SO4. H2O H2SO4 + 2H2O H2SO4. 2H2O Example: 1. Charring of sugar is another example of dehydration by sulphuric acid C12H22O11 (Sucrose) + H2SO4 12C + H2SO4. 11H2O 3. Oxidising Property: Hot conc. H2SO4 gives nascent oxygen in presence of reducing agent and hence it acts as an oxidizing agent. H2SO4 H2O + SO2 + (O) Examples: Oxidation of S : Conc H2SO4 oxidises S to SO2. [H2SO4 H2O + SO2 + (O)]x2 S + 2(O) SO2 2H2SO4 + S 2H2O + 3SO2 Action on Carbon: Conc.H2SO4 oxidises carbon to carbon dioxide. [ H2SO4 H2O + SO2 + (O) ] x 2 C + 2(O) CO2 2H2SO4 + C 2H2O + 2SO2 + CO2 Action on copper: When copper is heated with conc.H2SO4, it is oxidized to CuSO4. H2SO4 H2O + SO2 + (O) Cu + (O) CuO CuO + H2SO4 CuSO4 + H2O Cu + 2H2SO4 CuSO4 + 2H2O + SO2 Uses of Sulphuric acid:Sulphuric acid is called King of chemicals.The pattern of consumption of sulphuric acid is an index of industrial prosperity of any country. Some major uses of sulphuric acid are 1. For the manufacture of fertilizers, ammonium sulphate and superphosphate. 2. In petroleum refining to remove unsaturated compounds (which darken crude oil by air oxidation. 3. In the manufacture of HCl, HNO3,H3PO4, Metallic sulphates, alums, Na2CO3 etc. 4. In metallurgical process for the purification of metals. 5. In preparation of dyes, drugs and disinfectants. Chemistry p –BLOCK ELEMENTS Page 18 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 6. For cleansing metals before coating them with enamel, tin, Zinc this is called pickling. 7. In preparing paints and pigments. 8. In the manufacture explosives such as nitroglycerine, TNT, gun cotton. 9. In textile and paper industry 10. As a dehydrating agent. 11. In leather tanning. GROUP 17 ELEMENTS: Introduction: Fluorine, chlorine, bromine, iodine and astatine belongs to the 17 group elements of the periodic table. These are also called as halogens. Halogens are non-metallic elements and Astatine is a radioactive element. Occurrence: Fluorine is present as insoluble fluorides. (1) Fluorspar- CaF2 (2) Cryolite - Na3AlF6 (3) Fluorapatite – 3Ca3(PO4)2.CaF2. Small quantities of halogens are present in soil, river water plants, bones and teeth of animals. Sea water contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium but is mainly sodium chloride solution (2.5% by mass). Chlorine also occurs in the form of carnallite KCl.MgCl 2.6H2O. Various sea weeds contain up to 0.5% of iodine and Chile salt peter contains up to 0.2% sodium iodate. The trends of some physical and chemical properties of group 17 elements: Electronic Configuration Element Atomic Number Electronic configuration Name Symbol Fluorine F 9 [He] 2s22p5 Chlorine Cl 17 [Ne] 3s23p5 Bromine Br 35 [Ar] 3d104s24p5 Iodine I 53 [Kr] 4d105s25p5 Astatine At 85 [Xe] 4f145d106s26p5 The outer general electronic configuration of halogens can be represented as ns2 np5. 1. Atomic and Ionic Radii: Halogens have the smallest atomic radii in their respective period due to the highest effective nuclear charge in the halogen atoms. The atomic and ionic radii increase from F to I down the group due to the addition of a new electronic main shell to the atom or ion. 2. Ionisation enthalpy: Halogens have little tendency to lose electrons, i.e.,they have very high Ionisation enthalpy due to their small size and high effective nuclear charge. Ionisation enthalpy decreases down the group from F to I due to the increase in the atomic radii from F to I. 3. Electron gain enthalpy: Halogens have very high electron gain enthalpy [Electron gain enthalpy of halogens is negative] due to the small atomic size of halogens, halogens require only one electron to attain the stable configuration and the effective nuclear charge of halogens is very high. Electron gain enthalpy of fluorine is less negative than chlorine. Give reason. Fluorine has smaller negative electron gain enthalpy than chlorine, because Fluorine atom is very small. So, the electron density on fluorine is large, therefore the incoming electron experiences more repulsive force from the electron cloud of the F atom. So, the electron gain enthalpy of fluorine is smaller than that of chlorine. Down the group (F to I) the electron gain enthalpy decreases i.e., becomes less negative because of the increase in the atomic size. So, the force with which the added electron is attracted towards the atom is reduced. 4. Electronegativity: Halogens have high electronegativity. It decreases down the group down the group due to increase in size of the atom.Fluorine is most electronegative element in the periodic table. 5. Physical Properties: 1. “F and Cl” are gases, “Br” is a liquid and “I” is a solid. [The strength of intermolecular forces increases down the group.] 2. Halogens have low melting and boiling points. Chemistry p –BLOCK ELEMENTS Page 19 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 3. M.P. and B.P. of halogens increases from F to I due to increase in the strength of intermolecular forces (vander waal’s forces) of attraction from F to I. Here as atomic number increases heats of fusion as well as heat of vapourisation increase. 4. Fluorine has low enthalpy of dissociation than chlorine is due to the small size of F. So, F – F bond length in F2 is small. So, the charge density on fluorine atoms is very high and the repulsion between the non-bonding electrons is very high as compared to that of Cl2. 5. The enthalpy of dissociation decreases from Cl to I i.e., Cl – Cl > F –F > Br – Br > I – I. 6. Enthalpy of hydration of halides decreases down the group because of the increase in the atomic size. Que: Although electron gain enthalpy of Fluorine is less negative as compared to chlorine. Fluorine is stronger oxidizing agent. Why? Ans:This is due to (1) low enthalpy of dissociation of F – F bond (2) high hydration enthalpy of F. Chemical Properties: 1. Oxidation State: All halogens exhibit “-1” oxidation state. However, Chlorine, Bromine and iodine shows +1, +3, +5 and +7 oxidation states. Higher oxidation states of these elements are due to the presence of vacant d-orbitals. This can be explained as follows. The outer electronic configuration of halogens in ground state is ns2 np5. This results in –1 and +1 oxidation state. ↿ ↿ ↿ ↿ ⇂ ⇂ ⇂ ns2 np5 nd0 The outer electronic configuration of halogens in first excited state is ns2 np4 nd1. This results in +3 oxidation state. ↿ ↿ ↿ ↿ ↿ ⇂ ⇂ ns2 np5 nd1 The outer electronic configuration of halogens in second excited state is ns2 np3 nd2. This results in +5 oxidation state. ↿ ↿ ↿ ↿ ↿ ↿ ⇂ The outer electronic configuration of halogens in third excited state is ns1 np3 nd3. This results in +7 oxidation state. ↿ ↿ ↿ ↿ ↿ ↿ ↿ Fluorine exhibits only “-1” oxidation state because fluorine atom has no d-orbitals in its valence shell and therefore cannot expand its octet. 2. Reactivity of halogens: Halogens are the most reactive elements. Fluorine is the most reactive halogen, the reactivity of halogen decreases down the group as F2 > Cl2 > Br2 > I2. This is due to the decrease in electronegativity and increase in bond dissociation energy from F2 to I2. The high reactivity of halogens is due to the following reasons. 1. Low enthalpy of dissociation for halogens. 2. High electron gain enthalpy of halogens. 3. Oxidising nature: Halogens are strong oxidizing agents, because they are ready to accept the electrons. F2 is the strongest oxidizing agent in halogens. Halogens of low atomic number oxidize halide ions of higher atomic number. Fluorine oxidises Cl, Br, I, to chlorine, Bromine and Iodine. Ex: F2 + 2X 2F + X2 (Where, X = Cl, Br, I) – – Similarly chlorine oxidises Br and I to Br2 and I2. Cl2 + 2X ⟶2Cl + X2 (Where, X = I, Br) Bromine oxidises I – to iodine. Br2 + 2I ⟶ 2Br + I2 The oxidizing nature of halogens decreases down the group because the reduction potential of halogens decreases down the group. 4. Anomalous behavior of fluorine: The anomalous properties of fluorine are as follows. 1. Fluorine shows only -1 oxidation state, the other elements of 17 group shows +1, +3, +5, and +7 oxidation states. 2. Fluorine is more reactive than other elements. Chemistry p –BLOCK ELEMENTS Page 20 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 3. The bond enthalpy of F2 is lower than other elements. 4. HF shows a strong tendency towards hydrogen bonding but the remaining hydrogen halides do not form hydrogen bond. 5. Fluorine form only one oxoacid, the remaining elements form more than one oxoacids. 6. HF is a liquid due to strong hydrogen bonding remaining hydrogen halides are gases. 7. Most of the reaction of fluorine are exothermic due to small and strong bond formed by it with other elements. 8. Fluorine has high SRP ( Eo = +2.37V) Causes for anomalous behaviour of fluorine: The anomalous property of fluorine is due to a) Small atomic size b) Absence of d-orbital c) High ionization enthalpy and d) highest electronegativity. 5. Reactivity towards hydrogen:Halogens react with hydrogen to give hydrogen halide. The reactivity of halogens decreases from fluorine to iodine i.e., F2 > Cl2 > Br2 > I2 The acidic strength of hydrogen halides decreases from HF to HI i.e., HF < HCl < HBr < HI because the bond enthalpy decreases from HF to HI. Therefore H – I bond requires least and H – F bond requires the maximum energy. So HI is the strongest hydrohalic acid and HF is the weakest hydrohalic acid. Thermal stability: HF is the most stable, HI is the least stable hydrogen halide.HF>HCl>HBr>HI Because Thermal stability depends on the bond enthalpy. The halide having lesser bond enthalpy will be less stable. So, the thermal stability decreases from HF to HI because the bond enthalpy decreases from HF to HI. 2. Reactivity towards oxygen: Halogens form many oxides with oxygen but most of them are unstable. Fluorine forms two oxides OF2 and O2F2 but only OF2 is thermally stable.These are essentially oxygen fluorides because of higher electronegativity of fluorine than oxygen. Both are strong fluorinating agents. O2F2 oxidises plutonium (Pu) to PuF6. Chlorine, bromine and iodine form oxides in which the oxidation state of the halogen varies from +1 to +7.The stability of these oxides formed by halogens decreases from I > Br > Cl. The higher oxides of halogens tend to be more stable than lower oxides. Oxides of chlorine: Chlorine forms oxides like Cl2O, ClO2, Cl2O6 and Cl2O7. They are highly reactive oxidising agents and tend to explode. ClO2 is used as a bleaching agent for paper pulp and textiles and in water treatment. The bromine forms oxides like Br2O, BrO2 and BrO3 and are least stable and exist only at low temperatures. They are very powerful oxidising agents. The iodine forms oxides like I2O4, I2O5, and I2O7. They are insoluble solids and decompose on heating. I2O5 is very good oxidising agent and is used in the estimation of carbon monoxide. 3. Reactivity towards metals: Halogens react with metals to form metal halides. Example: Bromine reacts with magnesium to form magnesium bromide. Mg(s) + Br2 ⟶ MgBr2(s) The ionic character of halides decreases in the order MF > MCl > MBr > MI. If metal M exhibits more than one oxidation state the halides in higher oxidation state will be more covalent than the one in lower oxidation state. Example: SnCl4 is more covalent than SnCl2, PbCl4 more covalent than PbCl2, SbCl5 is more covalent than SbCl3and UF6 is more covalent than UF4. Chlorine: Preparation: 1. Chlorine may be prepared by the action of conc. HCl on KMnO4. 2KMnO4 + 16HCl 2KCl + 2MnCl2 + 8H2O + 5Cl2 2. Chlorine may be prepared by heating MnO2 with conc.HCl. MnO2 + 4HCl ⟶ MnCl2 + Cl2 + 2H2O. Chemistry p –BLOCK ELEMENTS Page 21 2015 TARALABALU SCIENCE ACADEMY SIRIGERE B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 Manufacture of Chlorine: 1. Deacon’s Process: In this process Cl2 is manufactured by the oxidation of HCl gas by atmospheric oxygen in presence of CuCl2 catalyst at 723K. CuCl2 4HCl + O2 → 2Cl2 + 2H2O. 2. Electrolytic Process: Chlorine can be manufactured by the electrolysis of brine (saturated NaCl solution). Upon electrolysis Cl2 is liberated at anode and H2 gas at anode. Properties: 1. Action of metals : Chlorine reacts metals like Al, Na, and Fe to form their respective chlorides. 2Al + 3Cl2 ⟶ 2AlCl3, 2Na + Cl2 ⟶ 2NaCl, 2Fe + 3Cl2 ⟶ 2FeCl3 2.Action of Non-metals: i) Chlorine reacts with sulphur S8 to form disulphur dichloride S2Cl2. S8 + 4Cl2 ⟶ 4S2Cl2 ii) Chlorine reacts with phosphorus P4 to form PCl3. P4 + 4Cl2 ⟶ 4PCl3. iii) It reacts with H2 gas to form HCl. H2 + Cl2 ⟶ 2HCl 3. Action of H2S: Chlorine oxidises H2S to form sulphur. H2S + Cl2 ⟶ 2HCl + S 4. Action of NH3: a) When chlorine reacts with excess of ammonia gives ammonium chloride and nitrogen. 8NH3 (Excess) + 3Cl2 ⟶ 6NH4Cl + N2 b) When excess of chlorine reacts with ammonia gives nitrogen tricloride. NH3 + 3Cl2(Excess) ⟶ NCl3 + 3HCl 5. Action of NaOH: a) When chlorine reacts with cold & dil. alkali gives a mixture of sodium chloride and sodium hypochlorite. 2NaOH + Cl2 NaCl + NaOCl + H2O b) When chlorine reacts with hot & conc. alkali gives a mixture of sodium chloride and sodium chlorate. 6NaOH + 3Cl2 5NaCl + NaClO3 + 3H2O 6. Action of Ca(OH)2: Chlorine reacts with slaked lime or calcium hydroxide gives bleaching powder. 2Ca(OH)2 + 2Cl2 Ca(OCl)2 + CaCl2 + 2H2O The composition of bleaching powder is Ca(OCl)2 . CaCl2 . Ca(OH)2 . 2H2O 7. Action of FeSO4: Chlorine oxidizes ferrous sulphate to ferric sulphate. 2FeSO4 + H2SO4 + Cl2 Fe2(SO4)3 + 2HCl 8. Action of Na2SO3: Chlorine oxidizes sodium sulphite to sodium sulphate. Na2SO3 + H2O + Cl2 Na2SO4 + 2HCl 9. Action of SO2: Chlorine oxidizes SO2 to sulphuric acid. SO2 + 2H2O + Cl2 ⟶ H2SO4 + 2HCl 10. Action of Iodine: Chlorine oxidizes Iodine to iodic acid. I2 + 5Cl2 + 6H2O ⟶ 2HIO3 + 10HCl. 11. Bleaching property: Chlorine acts as a powerful bleaching agent, bleaching action of chlorine is due to oxidation .Cl2 + H2O 2HCl + (O) Coloured substance +(O) Colourless substance 12. Action of Hydrocarbons: Chlorine reacts with hydrocarbons and gives substitution products with saturated hydrocarbons and addition products with unsaturated hydrocarbons. UV radiation Room temperature CH4 + Cl2 → CH3Cl + HCl C2H4 + Cl2 → C2H4Cl2 Chlorine water on standing loses its yellow due to formation of HCl and HOCl. Hypochlorous acid so formed gives nascent oxygen which is responsible for oxidising and bleaching properties of Cl2. Uses:1. It is used for bleaching woodpulp, bleaching cotton and textiles. 2. It is used in the extraction of gold and platinum. 3. It is used in the manufacture of dyes, drugs and organic compounds. Chemistry p –BLOCK ELEMENTS Page 22 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE 2015 4. It is used in sterilizing drinking water. 5. It is used in the preparation of poisonous gases such as phosgene (COCl2), tear gas (CCl3 NO2), mustard gas [ClCH2CH2 S CH2CH2 Cl]. Hydrogen Chloride: Laboratory preparation: When sodium chloride is heated with Conc. H2SO4 gives HCl 420 𝐾 NaCl + H2SO4 → NaHSO4 + HCl 823𝐾 NaHSO4 + NaCl → Na2SO4 + HCl HCl gas can be dried by passing through conc.H2SO4. Properties of HCl: 1. Acidic property: HCl dissolves in water and ionizes as HCl + H2O ⟶ H3O+ + Cl ; Ka = 107 High dissociation constant of water indicates that it is a strong acid in water. 2. Action of NH3: HCl gas reacts with NH3 gas to form white fumes of NH4Cl. NH3 + HCl ⟶ NH4Cl.(Ammonium chloride) 3. Action of Aqua regia: Three parts of conc. HCl and one part of conc. HNO3 are mixed, aqua regia is formed. This aqua regia dissolves noble metals like gold, platinum etc., Au + 4H+ + NO3 – + 4Cl – ⟶ AuCl− 4 + NO + 2H2O 3Pt + 16H+ + 4NO3 – + 18Cl – ⟶ 3PtCl2− 6 + 4 NO + 8H2O 4. Action of salts of weaker acids: HCl decomposes salts carbonates, bicarbonates and sulphites to liberate respective acidic oxides. Na2CO3 + 2HCl ⟶ 2NaCl + H2O + CO2 NaHCO3 + HCl ⟶ NaCl + H2O + CO2 Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 Uses of HCl: It is used 1. In the manufacture of chlorine, NH4Cl 2. In medicine as a laboratory reagent. 3. To prepare glucose from starch. 4. For extracting glue from bones. Oxoacids of Halogens: Fluorine forms only one oxoacids, hypofluorous acids (HOF) due to its small size and high electronegativity. The other oxoacids of halogens are HOCl - Hypochlorous acid. HOClO - Chlorous acid HOClO2- Chloric acid HOClO3- Perchloric acid H O O O O H Cl H , Cl , Cl O Hypochlorous acid Chlorous acid O Chloric acid H O Cl O O O Perchloric acid Interhalogen compounds: When two different halogens react with each other interhalogen compounds are formed. XX1, XX13, XX15, XX17 are the four different interhalogen compounds. Where, X – halogen of larger size, X1 – halogen of smaller size. Chemistry p –BLOCK ELEMENTS Page 23 TARALABALU SCIENCE ACADEMY SIRIGERE 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 As the ratio between radii of X and X1 increases the number of atoms per molecule also increases. Ex: Iodine (VII) fluoride (IF7) contains maximum number of atoms because the ratio of radii between I and F is maximum. Preparation of ClF: ClF may be prepared by the direct combination of chlorine with fluorine at 437K. 437K Cl2 + F2 → 2ClF Preparation of ClF3:ClF3 may be prepared by the direct combination of chlorine with excess fluorine at 573K 573K. Cl2 + 3F2 → 2ClF3 Preparation of ICl: ICl is prepared by mixing equimolar iodine and chlorine. I2 + Cl2 ⟶ 2ICl Preparation of ICl3: ICl3 is prepared by mixing iodine with excess chlorine. I2 + 3Cl2 ⟶ 2ICl3 Preparation of BrF3: BrF5 is prepared by action of Br2 diluted with water on F2. Br2 + 3F2 ⟶ 2BrF3. Preparation of BrF5: BrF5 is prepared by action of Br2 with excess of F2. Br2 + 5F2 ⟶ 2BrF5. Properties of Interhalogen compounds: All interhalogen compounds are covalent molecules and are diamagnetic in nature. They are volatile solids or liquids at room temperature except ClF which is a gas. Reactivity of interhalogen compounds compared to halogens: In general interhalogen compounds are more reactive than halogens (except fluorine). This is because X –X1 bond in interhalogen compounds is weaker than X – X bond in halogens except F –F bond. Hydrolysis of interhalogen compounds: Interhalogen compounds undergo hydrolysis to form halide ion derived from the smaller halogen and a hypohalite in XX1, halite in XX13, halate in XX15 and perhalate in XX17 derived from the larger halogen. XX1 + H2O ⟶ HX1 + HOX (Hypohalite) XX13 + 2H2O ⟶ 3HX1 + HOXO (Halite) XX15 + 3H2O ⟶ 5HX1 + HOXO2 (Halate) XX17 + 4H2O ⟶7 HX1 + HOXO3 (Perhalate) Molecular Shape of BrF3: Discuss the molecular shape of BrF3 on the basis of VSEPR theory. Solution: The central atom Br has seven electrons in the valence shell. Three of these will form electronpair bonds with three fluorine atoms leaving behind four electrons. Thus, there are three bond pairs and two lone pairs. Acc, to VSEPR theory. These will occupy the corners of a trigonal bipyramid. The two lone pairs will occupy the equatorial positions to minimize lone pair-lone pair repulsions which are greater than the lone pair – bond pair and bond pair – bond pair repulsions. The axial fluorine atoms will be bent towards the equatorial fluorine in order to minimize the lone pair-lone pair repulsions. Therefore the shape is slightly bent “T”. Uses: Interhalogen compounds may be used as; 1) Strong oxidizing agent. 2) In medicines. 3) Oxidizer for rocket propellants. 4) These compounds can be used as non-aqueous solvents. 5) Acts as strong fluorinating agent. Chemistry p –BLOCK ELEMENTS Page 24 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE GROUP 18 ELEMENTS: Helium, Neon, Argon, Krypton, Xenon and Radon belongs to 18 group of the periodic table. These 6 elements are also called as noble gases, because the valence shell orbitals of these elements have completely filled and therefore react with a few elements only under certain conditions. These elements are also called as Inert gases because of their chemical inertness (non-reactive under normal conditions). These gases are present in or around the earth in very small amounts so these are also called as rare gases. Occurrence: All noble gases except radon occur in the atmosphere. Their atmospheric abundance in dry air is ~1% by volume. Argon is the major component of these gases in the air. Xenon and radon are the rarest elements of the group. 222 4 Radon is produced during the radioactive disintegration of radium. 226 88𝑅𝑎 86𝑅𝑛 + 2𝐻𝑒 2 1. Electronic configuration: Except Helium (1s ) the outer electronic configuration of noble gases is ns2 np6. Element Atomic Number Electronic configuration Name Symbol Helium He 2 1s2 Neon Ne 10 [He] 2s22p6 Argon Ar 18 [Ne] 3s23p6 Krypton Kr 36 [Ar] 3d104s24p6 Xenon Xe 54 [Kr] 4d105s25p6 Redon Rn 84 [Xe] 4f145d106s26p6 2. Ionization enthalpy: Ionization energy of noble gases are very high because of the completely filled outer configuration but the ionization energy of 18 group elements decreases down the group because the atomic size increases down the group. 3. Atomic Radii: Atomic Radii increases down the group due to the addition of a new shell with each element in going down the group. 4. Electron gain enthalpy of noble gas elements are almost zero because all the shells of noble gas atoms are completely filled and therefore cannot accept any addition electron. Physical Properties: 1. All the noble gases are monoatomic and these are partially soluble in water. 2. Noble gases have very low melting and boiling points because the atoms of these elements are held together by weak dispersion forces (Vander waal’s forces) therefore, they are liquefied at very low temperature. 3. The melting and boiling points increases down the group, because the magnitude of Vander Waal’s force of attraction increases down the group. 4. Helium has the lowest boiling (4.2K) of any known substance. It has unusual property of diffusing through most commonly used laboratory materials such as rubber, glass or plastics. Chemical properties: Reason for chemical inertness: Noble gases are least reactive (Inert) because except helium the remaining elements have completely filled ns2 np6 configuration in their valence shell and the 18 group elements have high ionisation enthalpy and more positive electron gain enthalpy. Preparation of Xe Pt F6 [Xenon hexafluoroplatinate(V)] by Neil Bartlett: Neil Bartlett found that PtF6 reacts with oxygen to form O2+ [Pt F6]-. i.e. Pt F6 oxidises O2 molecule at room temperature. Since the ionization energy of O2 molecule (1175 KJ mol-1) is comparable to that of Xenon (1170 KJ mol-1). Bartlett argued that PtF6 should also react with Xenon to form similar compound Xe+ [ Pt F6]. In 1962, he prepared Xe[Pt F6] by mixing deep red vapours of Pt F6 with equal volume of Xenon. Xe + Pt F6 Xe [ Pt F6 ] Deep red vapours Chemistry Yellow solid p –BLOCK ELEMENTS Page 25 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE The compounds of krypton are fewer. Only the difluoride of krypton KrF2 has been studied in detail. Compounds of radon have not been isolated but only identified (e.g. RnF2) by radio tracer technique. No true compounds of Ar, Ne, or He are yet known. Preparation of XeF2: Xenon difluoride is prepared by the action of Xenon on fluorine at 673K under pressure of 1 bar using excess xenon 673K and 1bar Xe (g) + 3F2(g) → XeF6(s) Preparation of XeF4: Xenon tetrafluoride is prepared by the action of Xenon on fluorine at 873K under pressure of 7bar in 1:5 ratio. 573K,60−70bar Xe (g) + 3F2(g) → XeF6(s) Preparation of XeF6: 1. Xenon hexafluoride is prepared by the action of Xenon on fluorine at 573K under pressure of 60-70bar in 1:20 ratio. 573K,60−70bar Xe (g) + 3F2(g) → XeF6(s) 2. XeF6 can also be prepared by the action XeF4 on oxygen difluoride O2F2 at 143K. 143K XeF4 (g) + O2F2(g) → XeF6(s) + O2 Properties of Xenon-Fluorine compounds: XeF2, xeF4 and XeF6 are colourless crystalline solids and sublime readily at 298K. They are powerful fluorinating agents. Hydrolysis of XeF2: XeF2 undergo hydrolysis in presence of water to form Xe, HF and O2 2XeF2 + 2H2O ⟶2Xe + 4HF + O2 Structures of Xenon-Fluorine Compounds: Action of Fluoride ion acceptors: Xenon fluorides react with fluoride ion acceptors to form cationic species. XeF2 + PF5 ⟶ [XeF]+[PF6] – XeF4 + SbF5 ⟶ [XeF3]+[SbF6] – Action of Fluoride ion donors: Xenon fluorides react with fluoride ion donors to form anionic species. XeF6 + MF ⟶ M+[XeF7] – (M = Na, K, Rb or Cs) Preparation of XeO3: 1. When xenon hexafluoride undergoes hydrolysis xenon trioxide is formed. XeF6 + 3H2O ⟶XeO3 + 6HF 2. When Xenon tetrafluoride fluoride undergoes hydrolysis xenon trioxide is formed. 6XeF4 + 12H2O ⟶2XeO3 + 4Xe + 24HF + 3O2 Preparation of Xenon oxydifluoride (XeO2F2): When xenon hexafluoride undergoes partial hydrolysis xenon oxydifluoride is formed. XeF6 + 2H2O ⟶ XeO2F2 + 4HF Chemistry p –BLOCK ELEMENTS Page 26 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE Preparation of Xenon oxytetrafluoride (XeOF4): When xenon hexafluoride undergoes partial hydrolysis xenon oxytetrafluoride is formed. XeF6 + H2O ⟶ XeOF4 + 2HF Structures of Xenon-Oxygen Compounds: Application of Noble gases: The main use of noble gases is due to their chemical inertness and low boiling points. All noble gases find use in Lasers. Amongst noble gases helium and argon are easily available. Hence they are most widely used. Uses of Helium: 1) It is used in gas-cooled nuclear reactors as a heat transfer gas due to its following properties. a) High thermal conductivity b) Low viscosity c) Unaffected by radiation d) Inert nature 2) It is used for filling observation-balloons, due to its non-inflamability. Its lifting power is 92% of H2 gas 3) It is used as cryogenic fluid helping to produce and maintain the lowest temperature for scientific research. 4) It is used for providing an inert atmosphere. 5) A mixture of oxygen (80%) and helium (20%) is used as a respiration mixture by sea divers. If air is supplied to them, nitrogen of air dissolves in blood that causes a painful sensation called Caisson sickness or bends by bubbling out of blood when the diver moves from high pressure to atmospheric pressure. 6) Mixture of helium and oxygen is used to the treatment of asthma because it is lighter than air and hence defuses more rapidly through constricted lung passages. Uses of Neon: 1) Neon is largely used for producing neon lights. They consists of glass tubes filled with neon or a mixture of neon with other gases at low pressure ( 2mm of Hg) and glow on the passage of electric discharge.(Neon in a colourless tube produces orange red glow) 2) It is used in the beacon lights for pilots because these lights can penetrate through fog and mist also. 3) Neon tubes are used in various botanical gardens and green houses as it stimulates growth and effective in the formation of chlorophyll. 4) Neon is also used in television sets, spark plug testers, warning signals etc. Chemistry p –BLOCK ELEMENTS Page 27 2015 B Lingaiah Residential Pre-University College Sirigere - 577 541, Chitradurga Dist Tel: 08194-268824 TARALABALU SCIENCE ACADEMY SIRIGERE Uses of Krypton:1) It is used in high efficiency miner’s cap lamps. 2) Kr- 85 is used to measure thickness of sheets of metals and plastics. Uses of Argon:1. It is used for filling luminous sign tubes 2. The chief use of argon is in filling incandescent bulbs where it has following advantages over nitrogen a) Its thermal conductivity is less than that of nitrogen b) More inert than nitrogen and does not endanger the life of filament. c) Checks the volatilization of tungsten filament. d) It is light exerts negligible pressure on filament thus filament does not break soon. e) It is monoatomic and hence does dissociate even at high temperature hence no heat is lost in breaking atoms.3. A mixture of Ar and Hg vapour is used in fluorescent tubes 4. It is used for filling Geiger – Counter tubes. Uses of Xenon:1. It is used for filling radio and television tubes 2. It is used in bubble chambers for the detection of gamma rays and neutral mesons. 3. Xenon is used in discharge tubes for producing high speed flash of bluish light which is used in quick photography. Uses of Radon:1) In the preparation of ointment for treatment of cancer and other diseases. 2) Used in the scientific research on radioactivity. NOTE:1) Argon is the most abundant noble gas.2) Xenon is least abundant noble gas. 3) Helium is the lowest boiling liquid. 4) Helium has highest ionisation enthalpy. ■■■■ Chemistry p –BLOCK ELEMENTS Page 28