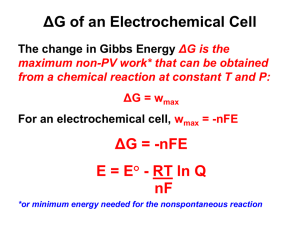
Thermochemistry (4 lectures)
advertisement

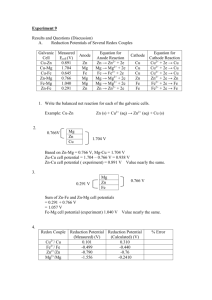
CHEM1612 - Pharmacy Week 9: Nernst Equation Dr. Siegbert Schmid School of Chemistry, Rm 223 Phone: 9351 4196 E-mail: siegbert.schmid@sydney.edu.au Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, John Wiley & Sons Australia, Ltd. 2008 ISBN: 9 78047081 0866 Electrochemistry Blackman, Bottle, Schmid, Mocerino & Wille: Chapter 12, Sections 4.8 and 4.9 Key chemical concepts: Redox and half reactions Cell potential Voltaic and electrolytic cells Concentration cells Key Calculations: Calculating cell potential Calculating amount of product for given current Using the Nernst equation for concentration cells Lecture 25-3 Recap: Standard cell potential The measured voltage across the cell under standard conditions is the standard cell potential E0cell (also called emf). Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s) Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) E0cell = E0cathode – E0anode = 0.34 - ( - 0.76)= 0.34 +0.76 = 1.10 V Lecture 26 - 4 Tricks to memorise anode/cathode 1. Anode and Oxidation begin with a vowels, Cathode and Reduction with consonants. 2. Alphabetically, the A in anode comes before the C in cathode, as the O in oxidation comes before the R in reduction. 3. Think of this picture: AN OX and a RED CAT (anode oxidation reduction cathode) Lecture 26 - 5 Standard cell potential and free energy and also ΔG0 < 0 and also ΔG0 > 0 For a spontaneous reaction, E0cell > 0 For a non-spontaneous reaction, E0cell <0 So there is a proportionality between E0 and -ΔG0. You also know that the maximum work done on the surroundings is -wmax = ΔG Electrical work done by the cell is w = Ecell × charge Lecture 26 - 6 Standard cell potential and free energy The emf E0cell is related to the change in free energy of a reaction: ∆G0 = –nFE0cell ∆G0 = Standard change in free energy n = number of electrons exchanged F = 96485 C/mol e- (Faraday constant) Also, away from standard conditions: ∆G = –nFEcell But what is Ecell? Lecture 26 - 7 Example Calculate ∆G0 for a cell reaction: Cu2+(aq) + Fe (s) Cu(s) + Fe2+ (aq) Is this a spontaneous reaction? Cu2+ + 2e– Cu E0 = 0.34 V Fe2+ + 2e– Fe E0 = –0.44 V E0cell = 0.34 - (-0.44) = 0.78 V ∆G0 = –nFE0cell ∆G0 = – 2 · 96485 · 0.78 = – 1.5 · 105 J This process is spontaneous as indicated by the negative sign of G0 and the positive sign for E0cell. Lecture 26 - 8 Example: a Dental Galvanic Cell Al(s)|Al3+(aq) || O2(aq), H+(aq), H2O(aq)|Ag,Sn,Hg Al is very easily oxidised, Al3+ + 3e− Al Eo = -1.66V. The filling is an inactive cathode for the reduction of oxygen, O2 + 4H+ + 4e− 2H2O and saliva is an electrolyte. Put the three together (biting on a piece of foil) results in generation of a current and possible pain. Lecture 26 - 9 Example: a Dental Galvanic Cell Al(s)|Al3+(aq) || O2(aq), H+(aq), H2O(aq)|Ag,Sn,Hg O2 + 4H+ + 4e– 2H2O E0 = 1.23 V Al3+ + 3e– Al E0 = –1.66 V 12H+ + 3O2 + 4Al 6H2O + 4Al3+ E0cell = 2.89 V ∆G0 = –nFE0cell= –12 · 96485 · 2.89 = –3346 kJmol–1 Lecture 26 - 10 But what is Ecell? Recall ΔG = ΔG0 + RT ln(Q) Since Image fromnobelprize.org Nernst Equation (1) ΔG0 = -nFE0cell and ΔG = -nFE Equation (1) becomes Walther Nernst Nobel Prize 1920 -nFE = -nFE0cell + RTln(Q) Q = [products] / [reactants] dividing both sides by –nF gives: E = E0 – RT ln(Q) nF Lecture 26 - 11 Nernst Equation Ecell = E0 – RT ln(Q) nF Since ln (x) = 2.303 log (x) E = E0 – 2.303 · RT log(Q) nF E = cell potential E0 = Standard cell potential R = Real Gas Constant = 8.314 JK-1mol-1 T =Temperature (K) n = no. of e- transferred F = Faraday constant = 96485 C mol-1 Q = Reaction quotient (Q = K at equilibrium) At 25°C, (2.303·R·298)/96485 = 0.0592 Ecell 0.0592 E log( Q) n 0 Nernst equation more commonly written like this (note: only at 25°C) Lecture 26 - 12 Example calculation 1 Calculate the expected potential for the following cell: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) E0 = 1.1 V i) [Cu2+] = 1.0 M; [Zn2+] = 10-5M ii) [Cu2+] = 10-5M; [Zn2+] = 1.0 M Ecell 0.0592 E log(Q) n 0 Firstly, work out the value of n : e- 2 mol transferred per mole of reaction: n = 2 Cu2+ + 2e- Cu Zn Zn2+ + 2e- Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Lecture 26 - 13 Example calculation Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Ecell 0.0592 0 E log(Q) n [Zn2+] (M) [Cu2+] (M) 1.0 1.0 10-5 1.0 (n=2) [Zn2 ] Q [Cu 2 ] Q log(Q) Ecell (V) 1.0 0.0 1.10 1.0 10-5 -5.0 1.25 10-5 105 5.0 0.95 Lecture 26 - 14 Demo: The effect of concentration 0.00 V Cu|Cu2+||Cu2+|Cu Both compartments of the voltaic cell are identical. E0cell = E0copper – E0copper = 0 (in standard conditions, 1M concentrations) What happens when the concentration of one cell is changed? Lecture 26 - 15 Demo: Cu Concentration Cell Low [Cu2+] Cu Cu2++ 2e- High [Cu2+] Cu2+ + 2e- Cu Cu |Cu2+||Cu2+|Cu E0 same for both half-reactions, so E0cell= 0. However, we have reduced the concentration of Cu2+ in one cell = nonstandard conditions. Electrical energy is generated until the concentrations in each half-cell become equal (equilibrium is attained). Can we explain this?… Add Na2S –precipitate forms. Lecture 26 - 16 Cu Concentration Cell Cu|Cu2+||Cu2+|Cu Low [Cu2+] Cu Cu2++ 2e High [Cu2+] Cu2+ + 2e- Cu E0cell is same on both sides, but the Cu concentrations are different. More charge carriers in one half-cell. If we poured the two solutions together, we would expect spontaneous mixing of two solutions of different concentrations to give one of equal concentration. The electrical connection allows electrons to pour from one half-cell to the other. Lecture 26 - 17 Concentration cells “Voltage” The measured cell potential in our experiment was “Voltage”. Let’s work out what the voltage should be: Cu Cu2+ (0.01 M) + 2eCu2+ (0.1M) + 2e- Cu Cu2+ (0.1M) Ecell E 0 Cu2+ (0.01 M) Ecell = “Voltage” 0.0592 y log(Q) 0.0 0.0296 log0.01 = “Voltage” n 0.1 Solve for “Voltage”: “Voltage” = 0.0296 V Lecture 26 - 18 Concentration cells The cell potential depends on the concentration of reactants. Corollary: It is useful to define a standard concentration, which is 1 M. Implication: We need to specify concentration when referring to the cell potential. The overall potential for the Cu/Cu2+ concentration cell is: E = E0cell – 0.0592/2 · log [Cu2+]dil / [Cu2+]conc Lecture 26 - 19 Reference Electrodes 1. Standard Hydrogen Electrode (SHE) 2. Metal-Insoluble Salt Electrode: Standard Calomel Electrode (SCE) and Silver Electrode 3. Ion-Specific: pH electrode Lecture 26 - 20 Standard Hydrogen Electrode (SHE) Finely divided electrode. surface Pt HCl solution with [H+] =1, H2 p= 1 atm bubbling over the electrode. H2 absorbs on the Pt, forming the equivalent of a 'solid hydrogen‘ electrode in equilibrium with H+. Platinum – gas electrode Metal – Metal ion Electrode H2 electrode Cu2+ + 2e- Cu Eo = 0.34 V H2 2H+ + 2 e- Eo = 0.00 V Pt|H2(g)|H+(aq)||Cu2+(aq)|Cu(s) Eocell = 0.34 – 0 = 0.34 V anode cathode Lecture 26 - 21 Metal-insoluble salt electrodes The Standard Hydrogen Electrode (SHE) isn't convenient to use in practice (can be contaminated easily by O2 or organic substances). There are more practical choices, like metal - insoluble salt electrodes. The potential of these electrodes depends on the concentration of the anion X- in solution. In practice 2 interfaces: X- + 1. M / MX insoluble salt: MX (s) + e- M (s) + X-(s) C X- 2. coating/solution: X- (s) X- (aq) C+ M C+ X- Overall: MX (s) + e-(metal) M (s) + X- (aq) C+ MX X - Lecture 26 - 22 Metal-insoluble salt electrodes MX The concentration of anions in solution is controlled by the salt's solubility: [Ag+] [X-] = Ksp X- C+ C+ XM C+ X- C+ X- Pt | Hg | Hg2Cl2 | KCl (1M) E 0 = 0.28 V Normal calomel electrode, Saturated calomel electrode Pt | Hg | Hg2Cl2 | KCl(sat.) E0 = 0.24 V Silver/Silver chloride, Ag | AgCl | Cl- (1M) E 0 = 0.22 V (used in pH meters) Lecture 26 - 23 Saturated Calomel Electrode The ‘saturated calomel electrode’ (SCE) features the reduction halfreaction: Hg+ + e– Hg Hg2Cl2 2Hg+ + 2Cl– Overall: Hg2Cl2 (s) + 2e– 2 Hg (s) + 2Cl– (sat) 5M Pt | Hg | Hg2Cl2 | KCl || Standard cell potential of E0 = 0.24 V. 2 Lecture 26 - 24 Cell Potentials 1 Q: The standard reduction potential of Zn2+/Zn is - 0.76 V. What would be the observed cell potential for the Zn/Zn2+ couple when measured using the SCE as a reference? Calomel: Hg2Cl2(s) + 2e- 2Hg(l) + 2Cl-(aq) E0 = 0.24V Zn Zn2+ + 2eE0 = + 0.76V (reversed because it is written as an oxidation) 0.24 Hg+/Hg 0.0V H2/H+ Ans: The Zn will be oxidised (lower reduction potential), so E (cell) = 0.76 + 0.24 = 1.00 V respect to the SCE So to get the oxidation half-reaction E0 using the SCE as cathode, subtract 0.24 V from the volt meter reading. -0.76 Zn2+/Zn Lecture 26 - 25 Summary CONCEPTS Concentration cells Nernst equation CALCULATIONS Work out cell potential from reduction potentials; Work out cell potential for any concentration (Nernst equation) Lecture 26 - 26 Silver electrode Ag+ + e– Ag AgCl Ag+ + Cl– Overall: AgCl + e– Ag + Cl– E0 = 0.22 V AgCl (s) + e– Ag (s) + Cl– (sat) A thin coating of AgCl is deposited on the pure metal surface. Ag | AgCl | Cl– Lecture 26 - 27 Cell Potentials 2 A Fe3+/Fe2+ cell with [Fe3+]=[Fe2+] =1 M has a potential of 0.55V respect to the Ag/AgCl electrode (E0= 0.22 V). What is the potential of this electrode with respect to the SHE? ? 0.22 Ag+/Ag Answer. The reactions that occur in the two half-cells are: Fe3+ + e- → Fe2+ at the cathode E = 0.55 V; E0 = ? Ag + Cl- +e-→ AgCl at the anode E0= 0.22 V 0.0V H2/H+ The potential of this electrode with respect to the SHE is the difference of the two electrode potentials: E = E0 - 0.22 E0Fe3+/Fe2+ = 0.55 + 0.22 = 0.77 V Lecture 26 - 28 Measurement of pH We could construct a concentration cell, using the standard hydrogen electrode (SHE) and a hydrogen electrode: unknown H2(g, 1 atm) 2H+(aq, unknown) + 2e- anode 2H+ (aq, 1M) + 2e- H2(g, 1 atm) cathode 2H+ (1M) 2H+ (unknown) Ecell = ? Using Nernst equation: 2 RT [H ]un RT 0 Ecell E ln 2 2 ln [H ]un 0.0592 pH nF 2F [H ]ref at 25°C, i.e. the measurement of the cell potential provides pH directly! Lecture 26 - 29 pH electrode The pH electrode potential is typically measured versus a fixed reference calomel electrode. Eglass electrode= E’ + RT/2.303F log [H+] E’ is the sum of the constant offset potentials of the inner glass surface/solution, the Ag/AgCl electrode, and the calomel electrode. • Based on a thin glass membrane: a modified glass enriched in H+ and resulting in a hydration layer a few micrometers thick. • Inside the membrane is a 'reference solution' of known [H+] (1M HCl). • The potential difference relevant to pH measurement builds up across the outside glass/solution interface marked ||. Lecture 26 - 30 Nernst Equation The Nernst equation describes the effect of concentration on cell potential. Ecell E 0 Q 0.0592 log( Q) n [products ] [reactants ] Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) When Q < 1, [reactants] >[products] and the cell can do more work. When Q = 1, Ecell = E0cell (standard conditions [x] = 1 M). When Q > 1 , [products] > [reactants] and Ecell is lower. Lecture 27 - 31 Difference between Q and K Figure from Silberberg, “Chemistry”, McGraw Hill, 2006. Breakdown of N2O4 to NO2: N2O4 (g, colourless) → 2 NO2 (g, brown) Q [ NO 2 ]2 [ N2O4 ] Q =K Lecture 27 - 32 Potential of an electrochemical cell Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Ecell (Volts) Ecell 0.0592 =E log( Q ) n 0 K [Zn 2 ] Q [Cu 2 ] 0V ~1037 Ecell decreases as the reaction proceeds, until at equilibrium Ecell =0 and E0 0.0592 log(K ) . n Lecture 27 - 33 Recap: Examining Q To K Ratios Ecell E 0 0.0592 log(Q) n If Q/K < 1 Ecell is positive for the reaction as written. The smaller the Q/K ratio, the greater the value of Ecell and the more electrical work the cell can do. If Q/K = 1, Ecell = 0. The cell is at equilibrium and can no longer do work. If Q/K > 1, Ecell is negative for the reaction as written. The cell will operate in reverse – the reverse reaction will take place and do work until Q/K = 1 at equilibrium. Lecture 27 - 34 Link between E 0 and K Q: What happens if the reaction proceeds until equilibrium is reached? A: The reaction stops, therefore the voltage, or electrical potential, is zero (the battery is flat). In mathematical terms: Ecell E 0 0.0592 log(Q) 0 n At equilibrium Q=K 0.0592 E log(K ) n 0 So the equilibrium constant determines the cell potential. Large K products favoured large standard cell potential, E0 Lecture 27 - 35 Redox reactions are special Figure from Silberberg, “Chemistry”, McGraw Hill, 2006. For redox reactions there is a direct experimental method to measure K and ΔG°. Lecture 27 - 36 Relation between E 0 and K Figure from Silberberg, “Chemistry”, McGraw Hill, 2006. 0.0592 E log(K ) n 0 K is plotted on a logarithmic scale to give a straight line. Lecture 27 - 37 Example question 2 Q: A voltaic cell consisting of a Ni/Ni2+ half-cell and Co/Co2+ half-cell is constructed with the following initial concentrations: [Ni2+] = 0.80 M; [Co2+]=0.2 M. a) What is the initial Ecell? b) What is the [Ni2+] when the voltage reaches 0.025 V? c) What are the equilibrium concentrations of the ions? Given: E 0 Ni2+/Ni = -0.25 V; E 0 Co2+/Co = -0.28 V Ni2+ + 2e- → Ni E 0 = -0.25V Co → Co2+ + 2e- E 0 = +0.28V Co(s) + Ni2+(aq) Co2+(aq) + Ni(s) E 0 = 0.03 V Lecture 27 - 38 Example question 2a a) What is the initial Ecell? Co(s) + Ni2+(aq) Co2+(aq) + Ni(s) E 0 = 0.03 V [Co2 ] 0.2 Q 0.25 2 [Ni ] 0.8 Ecell 0.0592 0.0592 E log(Q) 0.03 log(0.25) n 2 0 0.03 0.0296 (0.602) 0.048V Lecture 27 - 39 Example question 2b b) What is the [Ni2+] when the voltage reaches 0.025V? Ecell E 0 0.025 0.03 0.0296 log(Q) 0.0592 log(Q) n log(Q) Co(s) + Ni2+(aq) Co2+(aq) + Ni(s) 0.80 - x 0.005 0.169 0.0296 Q = 1.47 0.20+x [Co2 ] (0.2 x) Q 1.47 2 [Ni ] (0.8 x) [Co2+] = 0.60 M 1.176 1.47x 0.2 x 2.47x 0.976 So when Ecell = 0.025 V x = 0.40 [Ni2+] = 0.40 M Lecture 27 - 40 Example question 2c c) What are the equilibrium concentrations of the ions? 0.03 0.0296 log(K ) 0.0592 0E log(K ) n 0 log(K ) 0.03 1.014 0.0296 Co(s) + Ni2+(aq) Co2+(aq) + Ni(s) K = 10.24 0.80-x 0.20+x [Co2 ] (0.2 x) K 10.24 2 [Ni ] (0.8 x) 8.192 10.24x 0.2 x 11.24x 7.986 So at equilibrium, [Co2+] = 0.91 M x = 0.71 [Ni2+] = 0.09 M Lecture 27 - 41 Concentration Cells in Nature Concentration cells are present all around us, e.g. nerve signalling: concentration gradients produce electrical current ion pumps across cell membranes: Na+ / K+ pump, Ca2+ pump energy production and storage in cells: ATP Lecture 27 - 42 Nerve cells Energy from ATP hydrolysis is used by ion pumps, so that across the nerve cell membrane concentration gradients are maintained: Figure from Silberberg, “Chemistry”, Ion Concentration Gradient: McGraw Hill, 2006. Inside Outside K+ High Low Na+ Low High The membrane potential is more positive outside than inside the cell. On nerve stimulation, Na+ enters cell, the inside cell membrane becomes > +ive, then K+ ions leave cell to re-equilibrate the outside. These rapid (ms) changes in charge across the membrane stimulate the neighbouring region and the electrical impulse moves down the length of the cell. Lecture 27 - 43 Nerve cells Nernst Equation gives the membrane potential generated by the differing extracellular versus intracellular concentrations of each ion: Eion 2.303RT [ion]outside log nF [ion]inside Substituting n = 1, T = Eion 37oC: [ion]outside 61.5 log [ion]inside (Eo = 0) Consider K+: ( in mV) 1 mV = 10-3 V EK [K+]outside = 3 mM, [K+]inside = 135 mM [K ]outside 61.5 log [K ]inside 3 EK 61.5 log 135 61.5 1.65 mV 102 mV Lecture 27 - 44 Cellular Electrochemistry Biological cells apply the principles of electrochemical cells to generate energy in a complex multistep -O process. NH2 adenosine triphosphate O P O O O- P N O O O- P O O- Bond energy in food is used to generate an electrochemical potential. N N ATP O H N H H OH H OH H2O NH 2 The potential is used to create the bond energy of the high-energy molecule adenosine triphosphate (ATP) (energy currency for the cell). adenosine diphosphate O -O P O ATP4- + H2O → ADP3- + HPO42- + H+ ΔG °’ = -30.5 kJ mol-1 N O O - N P O O - O H H H OH H OH N N ADP + HPO42- + H+ ΔGo’ (solution at pH 7 and at human body temperature 37oC.) Lecture 27 - 45 Bond Energy to Electrochemical Potential Inside mitochondria, redox reactions are performed by a series of proteins that form the electron-transport chain (ETC) which contain redox couples, such as Fe2+/Fe3+. Large potential differences provide enough energy to convert ADP into ATP. Lecture 27 - 46 Figure from Silberberg, “Chemistry”, McGraw Hill, 2006. Bond Energy to Electrochemical Potential In nature, the most important reducing agent is a complex molecule named nicotinamide adenine dinucleotide, abbreviated NADH, which functions as a hydride donor (H-). = NADH = biological reducing agent NAD+ = biological oxidising agent Lecture 27 - 47 Electron Transport Chain (ETC) ETC consists of three main steps, each of which has a high enough potential difference to produce one ATP molecule. The reaction that ultimately powers ETC is the reduction of oxygen in the presence of NADH: 2H+ + 2e- + ½ O2 → H2O Eo’ = +0.82 NADH + H+ → NAD+ + 2H+ + 2e- Eo’ = -0.32 NADH(aq) + H+(aq) + ½O2(aq) → NAD+(aq) + H2O(l) Eo’overall = 1.14 V ΔGo’ = -nFEo’ = -2 · 96485 C mol-1· 1.14 V = - 220 kJ mol-1 Substantial energy release! Lecture 27 - 48 ATP Synthesis In short: e- are transported along the chain, while protons are forced into the intermembrane space. This creates a H+ concentration cell across the membrane. In this step, the cell acts as an electrolytic cell, i.e. uses a spontaneous process to drive a non-spontaneous process. Figure from Silberberg, “Chemistry”, McGraw Hill, 2006. When [H+]intermembrane/[H+]matrix ~ 2.5 a trigger allows protons to flow back across membrane, and ATP is formed. It’s not simple: Noble prize in 1997 to Boyer and Walker for elucidating this. Lecture 27 - 49 Summary CONCEPTS Concentration cells Nernst equation E 0 and K Link between E , Q and K Applications of concentration cells CALCULATIONS Work out cell potential from reduction potentials; Work out cell potential for any concentration (Nernst equation) Work out K from E 0 Work out pH from concentration cell Lecture 27 - 50 Pop Quiz 1 Balance the following reaction in basic solution: MnO4- + CN- MnO2 + CNO- Answer: H2O + 2 MnO4- + 3 CN- --> 2 MnO2 + 3 CNO- + 2 OH- Lecture 26 - 51 Pop Quiz 2 Balance the following reaction in basic solutions: NO3- + Zn Zn2+ + NH3 Answer: NO3- + 4 Zn + 6 H2O → 4 Zn2+ + NH3 + 9 OH- Lecture 26 - 52