Heat and Temperature: A Physics Presentation
advertisement

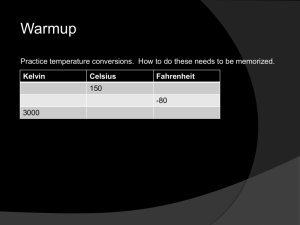
Heat is a form of: Everything in the universe has heat energy! Your BODY, your CAR…even ICE! Temperature: Temperature is ONLY a measurement of particle motion. Temperature measures the average kinetic energy of the molecules of an object or substance. SUMMARY: HEAT IS ENERGY TEMPERATURE IS A MEASUREMENT OF MOTION 3 Main Temperature Scales 1) Celsius 2) Fahrenheit 3) Kelvin What you need to know about the Fahrenheit Temperature Scale 1) Fresh water freezes at 32 degrees Fahrenheit 2) Fresh water boils at 212 degrees Fahrenheit What you need to know about the Celsius Temperature Scale 1) Fresh water freezes at 0 degrees Celsius 2) Fresh water boils at 100 degrees Celsius 3) It is the most commonly used scale except for in the United States. What you need to know about the Kelvin Temperature Scale 1) There are NO NEGATIVE NUMBERS 2) Based on absolute zero (all molecule movement has stopped). 3) The size of a Kelvin degree is equal in magnitude (size) to a Celsius degree. Temperature is measured with thermometers. There are several types of thermometers. THERMOMETER TYPES: 1) Liquid Thermometer 2) Bimetallic strip 3) Digital Liquid Thermometer Can only measure temperatures in a certain range. Uses the expansion of liquid alcohol or mercury (Hg) to indicate changes in temperature. Bimetallic Strip A coil is made using two different metal strips pressed together Both strips expand and contract at different rates as the temperature changes As the temperature changes, the coil winds and unwinds DIGITAL THERMOMETERS Measures temperature by noting the change in current Changes in temperature also cause electric current to change in a circuit Heat moves from objects with higher energy to objects with lower energy HEAT CAN MOVE IN 3 WAYS 1) CONDUCTION 2) CONVECTION 3) RADIATION For heat to be transferred by conduction: objects must be in direct contact with each other— THEY MUST BE TOUCHING!!! Convection: Transfer of energy by the movement of fluids with different temperatures For it to be fluid, it can be a liquid or a gas! Radiation: Transfer of energy by electromagnetic waves NO DIRECT CONTACT!!! Does not require a medium to travel! It is the only way solar heat energy can travel to Earth! Some objects act as conductors and some act as insulators. A conductor is a material that transfers heat quickly An insulator is a material that slows the transfer of heat Specific Heat Describes how much energy is required to raise an object’s temperature. The specific heat value is how much energy is required to raise 1 kg. of a substance by 1 degree Kelvin or Celsius Denoted by “c” in calculations. Specific Heat Equation Energy = (specific heat) x (mass) x (temperature change) OR energy = cm t The Kinetic Theory of Matter explains the behavior of molecules in matter. It states that all matter is made of constantly moving particles that collide without losing energy. When the temperature of a substance is increased, its molecules move faster and usually move farther apart. This is thermal expansion. It occurs in all forms of matter (there are a few exceptions). Water is an exception – it expands when it is cooling from 4 degrees C to 0 degrees C. There are four states of matter: 1. Solid State 2. Liquid State 3. Gas State 4. Plasma State For Solids: • Particles are packed closely together and are constantly vibrating in place • Solids have a fixed volume • Solids have a fixed shape For Liquids • The attractive forces between particles are weaker than in a solid • Particles can slide past each other • Liquids have a fixed volume. • Liquids do not have a fixed shape. For Gases • Particles are farther apart than in a liquid or solid. • Attractive forces are weak. • Gases have no definite shape. • Gases have no definite volume. For Plasma • Most common state of matter in the universe • Consists of positively and negatively charged particles. • No definite shape • No definite volume Changes in the thermal energy of a material can cause it to change from one state to another. Melting – When thermal energy is added to a solid, the change of a substance from a solid to a liquid is called melting. Heat of fusion – The amount of energy required to change 1 kg of a substance from a solid to a liquid at its melting point, or when a liquid becomes a solid. Freezing – Phase change of an object from the liquid phase to the solid phase. Vaporization – Change of a liquid into a gas. 1. Evaporation-Vaporization that occurs at the surface of a liquid. 2. Boiling – Vaporization occurring throughout the liquid. Condensation – When a gas changes into a liquid. To raise indoor air temperature on a cold day, energy must be transferred into a room’s air by a heating system. Heating system – Any device or process that transfers energy to a substance to raise the temperature of the substance. Types of Heating Systems: Forced Air Systems Radiator Systems Electric Heating Systems Heating and Work increase thermal energy You can warm your hands by either placing them near a heat source or rubbing them together In this example, your hands would be considered to be a system. A system can be a group of objects you can draw a boundary around to consider certain values about a scenario. THERE ARE 2 TYPES OF SYSTEMS: OPEN-Thermal energy can flow across your “boundary” or if work is done across the boundary CLOSED – Thermal energy is contained within the “boundary” and no outside work is done. First Law of Thermodynamics The increase in the thermal energy of a system equals the work done on the system plus the thermal energy transferred to the system Second Law of Thermodynamics Energy moves from warmer objects (higher energy) to cooler objects (lower energy) Cooling System – A device that transfers energy as heat out of an object to lower its temperature. Cooling Systems use evaporation to transfer energy from their surroundings using a refrigerant Refrigerant – A substance used in cooling systems that transfers large amounts of energy as it changes state

![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)