Carbohydrates
advertisement

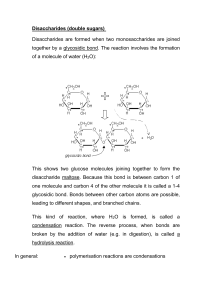
Carbohydrates Discussion Questions: (Groups of 2 – 5 minutes!!!) List some food items rich in carbohydrates. What is the difference between complex carbohydrates versus simple carbohydrates? Would you recommend a person who wants to lose weight to adopt a low-carb diet? Canada’s Food Guide Carbohydrates make up the largest volume of our daily food Carbohydrates are found in: grain flour, cereals, pasta, potatoes and other vegetables, fruits, syrups, honey, candy, pop, juice, milk as well as in the pure crystalline form of our familiar table "sugar". Functions of Carbohydrates Energy source Plays a vital part in the digestion, metabolism, and oxidation of protein and fat. Too much carbohydrate = Glycogen in liver OR Fat Simple versus Complex Carbohydrates Simple Carbs: Quick source of energy (do not supply other nutrients or fiber) Quickly absorbed – rapid increase in blood sugar level followed by quick decline Small molecules (glucose, fructose, maltose, sucrose, lactose) Found in: fruit, fruit juice, table sugar, honey, soft drinks, and other sweets Complex Carbs: Supply energy and other nutrients and fiber Slowly digested – steady slow rise in blood sugar level Large molecules (starch and dietary fiber) Starch: breaks down into smaller carbs; supplies long, sustained energy to the body bread, cereal, potatoes, pasta, rice, and legumes (dried peas and beans) Dietary fiber: found in plant cells (non-digestible part of plants) tough and stringy it does not break down completely in the body Essential for regulating the body (digestion). bran, whole-grain foods, raw vegetables and fruit (especially the seeds and skins), legumes, nuts, seeds and popcorn Some General Chemical Info Hydrophilic organic molecule General formula (CH2O)n , n = number of carbon atoms for glucose, n = 6, so formula is C6H12O6 Names of carbohydrates word root sacchar- or the suffix -ose often used monosaccharide or glucose Aldoses and Ketoses Aldoses contain the aldehyde group Ketoses contain the ketone group Ring Formation In aqueous solution, the aldehyde and ketone groups react with hydroxyl (-OH) group belonging to the same molecule Monosaccharides • Simplest carbohydrates • Mono = one, Saccharide = sugar • Glucose, Galactose, and Fructose are examples of monosaccharides • They are all isomers of each other because they have the same molecular formula (C6H12O6) but different structures Sugar Derivatives The hydroxyl groups of a simple monosaccharide can be replaced by other groups Alpha (a) and Beta (b) Links The hydroxyl group on the carbon that carries the aldehyde or ketone can rapidly change from one position to the other b a Disaccharides Pairs of monosaccharides Three major disaccharides sucrose lactose glucose + fructose glucose + galactose maltose glucose + glucose Dehydration Synthesis of a Disaccharide Dehydration synthesis of two glucose molecules results in the formation of maltose The C-O-C bond formed is called a glycosidic bond Polysaccharides Starch, cellulose and glycogen long chains of glucose form these polysaccharides Starch produced by plants is digested by amylase Cellulose gives structure to plants, fiber to our diet Starch Hydrolysis The digestion of starch occurs by the hydrolysis of the glycosidic bond. Amylase is the enzyme. Maltose is the product. Polysaccharides Glycogen is an energy storage polysaccharide produced by animals Liver cells synthesize glycogen after a meal to maintain blood glucose levels Carbohydrate Functions Source of energy Conjugated carbohydrates glycolipids external surface of cell membrane glycoproteins external surface of cell membrane mucus of respiratory and digestive tracts Moieties of Macromolecules A moiety is a chemically different component of a conjugated macromolecule Glycolipid glycoprotein