Title
advertisement

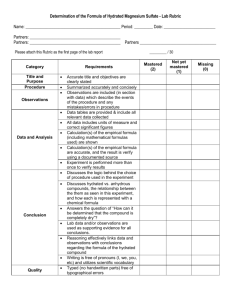
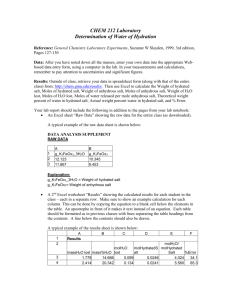
Title: Percent Composition of a Hydrated Crystal & Empirical Formula of a Hydrate (Lab Guidelines: 1,2,3,4,5,6,7,9,10,11,12) Purposes: 1) To determine the percent water (by mass) in a hydrated crystalline ionic compound. 2) To determine the empirical formula of the hydrated ionic compound. Background Information: Some ionic compounds naturally absorb water from the air to form hydrates. Formulas for the hydrated crystals are written in the following manner: MgSO47H2O. This means that for every one formula unit of MgSO4 there are 7 water molecules incorporated into the crystalline structure. The water molecules are physically attached to the structure, such that for every 1 mole of MgSO4 present, there are 7 moles of water present. Now, determine the % composition of water in MgSO47H2O. Today we will be working with CuSO4XH2O in order to experimentally determine: 1) the percentage of water by mass in this compound; and 2) the value of “X.” What simple process will we use in order to solve these 2 problems. What data will we need to record in order to be able to calculate the percentage of water, and the value of X? Materials: balance, scoopula, evaporating dish, watch glass, ring stand, ring clamp, wire gauze, hydrated copper (II) sulfate, Bunsen burner. Procedure: Write a step by step procedure in which you underline each of the pieces of equipment and materials as you mention them. Hints: Use about 2 grams of solid. Heat for approximately 5 minutes. Find the mass of the evaporating dish and watch glass only after they have cooled. Do not mass them while hot!! Just for fun: after the lab, add drops of water one at a time to the dehydrated salt in order to re-hydrate it. Observe what happens. Dispose of waste in the sink with lots of water. Data: What important pieces of data will you need? Don’t forget anything. See Table below. Mass of evaporating dish + watch glass Mass of evaporating dish + watch glass + hydrated salt before heating Mass of evaporating dish + watch glass + dehydrated salt after heating Calculations: Show them clearly. Label what you are calculating. Use appropriate units. Circle answers! Show calculations for Purpose 1 and Purpose 2 in separate sections. Determine % water and X value to the appropriate number of significant figures! Questions Answer the following questions by showing appropriate work and circling your answer: 2.08 g of BaCl2XH2O is heated in order to drive off the water. 1.78 g of dehydrated BaCl2 remains after heating. 1. What percentage of the original hydrated salt is water? 2. What is the value of X? 3. Write the formula of the hydrated salt. Make sure you show all necessary work. Error Analysis: After you are given the actual value of “X”: 1) Calculate the actual percentage of water in the hydrated crystal (show work). 2) Calculate 2 percent errors. a) Percent error for your % water calculation. b) Percent error for you calculated “X” value. Discuss possible errors and what impact they could have had on your results. Conclusion: Write a conclusion which demonstrates that you understand what you did. How did you accomplish the purposes. Discuss the math as well. Remember, here is a great guide for writing your conclusion: 1) Restate the purpose 2) Summarize the procedure and data collection 3) Discuss any important principles / equations used 4) Explain the graphs and/or math 5) Report your results Also, please include any other interesting information you would like to include. Lab Guidelines: 1,2,3,4,5,6,7,9,10,11,12