WATKINS - Chabot College
advertisement
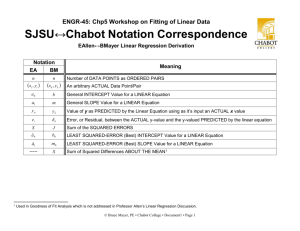
Engineering 45 Crystalline MicroStructure Bruce Mayer, PE Registered Electrical & Mechanical Engineer BMayer@ChabotCollege.edu Engineering-45: Materials of Engineering 1 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Learning Goals Learn How atoms assemble into solid structures • Use metals as Prototypical Example Determine Relationship Between Material density and material MicroStructure Understand how material properties vary with the sample (i.e., part) orientation Engineering-45: Materials of Engineering 2 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Properties of Solid Materials Mechanical: Characteristics of materials displayed when Forces and/or Moments are applied to them. Physical: Characteristics of materials that relate to the interaction of materials with various forms of Energy. Chemical: Material characteristics that relate to the e− structure of a material. Dimensional: Size, shape, and finish Engineering-45: Materials of Engineering 3 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Material Properties Chemical Physical Mechanical Dimensional Composition Microstructure Phases Grain Size Corrosion Melting Point Thermal Magnetic Electrical Optical Tensile properties Toughness Ductility Fatigue Hardness Standard Shapes Standard Sizes Surface Texture Stability Mfg. Tolerances Crystallinity Molecular Wt Flammability Acoustic Gravimetric Creep Compression Engineering-45: Materials of Engineering 4 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Energy and Atomic Packing NonDense, Random Packing Energy typical neighbor bond length typical neighbor bond energy Dense Regular Packing r Energy typical neighbor bond length r Regular Structures typical neighbor bond energy Tend to have LOWER Energy → Energetically Favored Engineering-45: Materials of Engineering 5 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Materials and Atomic-Packing CRYSTALLINE Materials • atoms pack in periodic, 3D arrays • typical of: Metals, many Ceramics, and some Polymers crystalline SiO2 NONcrystalline Materials • atoms have no periodic packing • occurs for: – Complex structures – Rapid Cooling "Amorphous" Noncrystalline Engineering-45: Materials of Engineering 6 noncrystalline SiO2 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Crystal Basics Crystalline Material • atomic arrangement in solid: – periodic and repeating 3D array – Long Range Order Unit Cell Smallest Repeating Entity Within a Lattice • Geometry lattice Engineering-45: Materials of Engineering 7 – Lattice Constants: a, b, c – interaxial angles: a, b, g Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Crystal Structure Crystal Structure geometry + atom-positions • i.e.; the spatial arrangement of Atoms, Ions, or Molecules Some Types Crystal Type Face Centered Cubic (FCC) Body Centered Cubic (CCC) B Hexagonal Close Packed (HCP Typical Metal Typical Ceramics Cu, Al Cr, W Ti, Zn NaCl CsCl ZnS polymer Engineering-45: Materials of Engineering 8 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Example Crystal Systems Tab 3.2 In Text Shows the 7 Most Common Crystal Systems Hexagonal • a=bc • a = b = 90°, g = 120° • Some Examples Cubic • a=b=c • a = b = g = 90° Engineering-45: Materials of Engineering 9 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Simple Cubic Stucture (SC) Rotate Rare due to poor packing (only Po has this structure) Close-packed directions are cube edges. Coordination No. = 6 • CoOrd No. (CN) = the No. of Nearest Neighbors Engineering-45: Materials of Engineering 10 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt HardSphere Model Reduced Sphere Mod Lattice Constants InterAxial 's a (pm) b (pm) c (pm) a b g 335.9 335.9 335.9 90 90 90 Note: 100 PicoMeters = 1 Å Engineering-45: Materials of Engineering 11 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt http://www.webelements.com/webelements/index.html http://www.webelements.com/webelements/elements/text/Po/key.html http://www.webelements.com/webelements/elements/text/Po/xtal.html Polonium, Z = 84, SC Structure Atomic Packing Factor, APF Assuming HardSphere Model Volume of ATOMS In a Unit Cell APF TOTAL Volume of Unit Cell APF For Simple Cubic Structure = 52% atoms unit cell a R=0.5a close-packed directions contains 8 x 1/8 = 1atom/unit cell Engineering-45: Materials of Engineering 12 APF = volume atom 4 p(0.5a) 3 1 3 a3 volume unit cell Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Body Centered Cubic (BCC) HardSphere Model Atoms per Unit Cell = 1 + (8 x 1/8) = 2 CoOrd No. = 8 Rotate Engineering-45: Materials of Engineering 13 • 8 Atoms Touch the “Center” Atom Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Atomic Packing Factor: BCC Find Radius, r, in terms of Lattice Const, a Thus r in Terms of a 3 3a 4r r a 0.433a 4 And Vsphere = (4/3)pR3 So the BCC APF Atoms Touch On Cube Diagonal, L • L = a3 = L = 4r Engineering-45: Materials of Engineering 14 4 3 3 3 2 at cell p a m at 3 4 APF a 3 m3 cell APFBCC 68.02% Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Face Centered Cubic (FCC) HardSphere Model Atoms per Unit Cell = 6x½ + (8 x 1/8) = 4 CoOrd No. = 12 Rotate Engineering-45: Materials of Engineering 15 • CN = 4top + 4bot + 4mid Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Atomic Packing Factor: FCC Find Radius, r, in terms of Lattice Const, a Thus r in Terms of a 2 a 4r r 1 2 2 a 0.3536a And Vsphere = (4/3)pR3 So the FCC APF Atoms Touch on Face Diagonal, f • f = a2 = 4r Engineering-45: Materials of Engineering 16 4 1 3 3 4 at cell p a m at 3 2 2 APF a 3 m3 cell APFFCC 74.05% Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt FCC Stacking Sequence An ABCABC... Stacking Sequence • The 2D projection A sites B sites B A B C B C B B C B B C sites The FCC Unit Cell Engineering-45: Materials of Engineering 17 A B C Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Hexagonal Close Packed (HCP) Built Up in an A-B Stacking Pattern Exhibits NonCubic Symmetry on a & c axes • c:a Ratio 1.633 Atoms per Cell = 6 • (12 x 1/6)corners + (2 x ½)top/bot + 3mid = 6 c APF and CN are the SAME as the FCC Structure • APF = 74.05% a Engineering-45: Materials of Engineering 18 – Close Packed Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt HCP CoOrdination Number For 3 Stacking Planes Below, Consider the CENTER Atom Observe The Center Atom’s Nearest Neighbors • 6 Surrounding in the Center Plane • 3 Touching From Below • 3 Touching From Above Thus CN HCP 3 6 3 12 Engineering-45: Materials of Engineering 19 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Structure of Compounds Compounds: Often have similar closepacked structures Expand Na+ ions to Reveal Close-Packed X-tal Structure NaCl Structure • Ionic Radii – Na+ = 116 pm – Cl– = 167 pm Engineering-45: Materials of Engineering 20 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Theoretical Density, r Atomic Radii for Crystals are Measured by X-Ray Diffraction • Can use The Data From XRD Measurements to Calc Density for Crystals Fcn of Ratom On the Macro Scale Densities are Calc’d by Weight & Measure of Chunks of Crystals Engineering-45: Materials of Engineering 21 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Theoretical Density Example For Copper FCC Cu has 4 atoms per Unit Cell; so r • Ratom = 128 pm • Acu = 63.54 g/mol nACu r VC N A • Xtal Structure = FCC 4 63.54 3 16 2 128pm 6.023 10 23 Recall From FCC APF Calc R 1 2 2 r 8.893 10 30 g/pm 3 8893 kg/m 3 a a 2 2R rmacro = 8940 kg•m-3 And The VC = a3 3 a 2 2R 8 2 2 R 3 Engineering-45: Materials of Engineering 22 3 • 0.53% HIGHER than Theoretical Value Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Characteristics of Some Elements at 20 °C Density At. Weight Element Symbol (amu) Aluminum Al 26.98 Argon Ar 39.95 Barium Ba 137.33 Beryllium Be 9.012 Boron B 10.81 Bromine Br 79.90 Cadmium Cd 112.41 Calcium Ca 40.08 Carbon C 12.011 Cesium Cs 132.91 Chlorine Cl 35.45 Chromium Cr 52.00 Cobalt Co 58.93 Copper Cu 63.55 Flourine F 19.00 Gallium Ga 69.72 Germanium Ge 72.59 Gold Au 196.97 Helium He 4.003 Hydrogen H 1.008 Engineering-45: Materials of Engineering 23 (g/cm3) 2.71 -----3.5 1.85 2.34 -----8.65 1.55 2.25 1.87 -----7.19 8.9 8.94 -----5.90 5.32 19.32 ----------- Atomic radius (nm) 0.143 -----0.217 0.114 ----------0.149 0.197 0.071 0.265 -----0.125 0.125 0.128 -----0.122 0.122 0.144 ----------15 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Densities Of Material Classes rmetals > rceramics > rpolymers Why? Metals have... • close-packing (metallic bonding) • large atomic mass Ceramics have... • less dense packing (covalent bonding) • often lighter elements Polymers have... • poor packing (often amorphous) • lighter elements (C,H,O) Composites have... • intermediate values Engineering-45: Materials of Engineering 24 Data from Table B1, Callister 6e. Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt 16 SINGLE Crystal Applications Most Crystalline Materials Are Composed of many Small Crystals • i.e., they are POLYcrystalline and exhibit a GRAIN Structure Grain Structure Introduces Weakness into the Material Engineering-45: Materials of Engineering 25 But Making LARGE Single Crystals is Very Difficult; i.e., Expensive Single Xtal Examples • SemiConductor wafers • Turbine Blades Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt 1-Material; Several Crystal Types Polymorphism More Than One Crystal Structure • Often Found in Compounds Allotropy Polymorphism in ELEMENTAL Solids Examples • Carbon Allotropes – Graphite – Diamond – Bucky Balls/Tubes Engineering-45: Materials of Engineering 26 • Iron Allotropes – BCC Ferrite (RT) – FCC Austenite (> 912 °C) – BCC Delta (~TMelt) Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Cabon Allotropes Graphite – Layers of Hexagonally Bonded C-atoms Diamond - tetrahedral, covalent bonds, SingleElement Form; the ZincBlende Crystal structure C60 Fullerenes Engineering-45: Materials of Engineering 27 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt WhiteBoard Work Example similar to P3.15 → FIND APF • Uranium • Orthorhombic • Lattice Constants – a = 286 pm – b = 587 pm – c = 495 pm • ratom = 138.5 pm • r = 19050 kg/m3 • AU = 238.03 g/mol Engineering-45: Materials of Engineering 28 SR Case Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Uranium Unit Cell (BCR) Engineering-45: Materials of Engineering 29 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Uranium Unit Cell (BC) Engineering-45: Materials of Engineering 30 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Uranium Unit Cell (SR) Engineering-45: Materials of Engineering 31 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt All Done for Today Uranium has the Highest Z Of Any Natural Element Engineering-45: Materials of Engineering 32 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Engineering-45: Materials of Engineering 33 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt P3-15 Engineering-45: Materials of Engineering 34 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Engineering-45: Materials of Engineering 35 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt Engineering-45: Materials of Engineering 36 Bruce Mayer, PE BMayer@ChabotCollege.edu • ENGR-45_Lec-03_Crystal_Structure.ppt