May The Forces be With You
advertisement

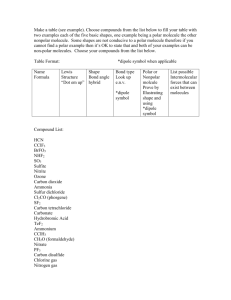
Name: _____________________________________________ Assignment #: _____ Due: __________ May The Forces be With You Part 1: Draw the following Lewis structures and circle best answers below each structure. H2O – Water CH3(CH2)4CH3-Hexane CH3CH2OH-Ethanol CH3CCH3-Acetone Polar Bonds Y or N? Polar Bonds Y or N? Polar Bonds Y or N? Polar or Nonpolar Molecule Polar or Nonpolar Molecule Polar or Nonpolar Molecule Polar Bonds Y or N? Polar or Nonpolar Molecule Part 2: Up, Up, and Away … Take Off (Evaporation Rate) 1) Obtain a piece of cotton and a rubber band. 2) Wrap cotton around tip of temperature probe and secure with rubber band. 3) Dip probe into vial of chemical to be studied (water, hexane, ethanol, and acetone) and wait for temperature to stabilize. This temperature will be your temperature data for 0.0 minutes. 4) Carefully remove probe from vial (put cap on vial) and place probe on bench such that probe is hanging over counter. You can secure it with a piece of masking tape. 5) On a separate sheet of paper, record temperature data points every 30 seconds. Collect data for a total of 10 minutes. When done discard cotton and rubber band in garbage. 6) Repeat procedure for other three liquids. Time (min.) Acetone (oC) Ethanol (oC) 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 9.5 10.0 Water (oC) Hexane (oC) Name: _____________________________________________ Assignment #: _____ Due: __________ For your lab report, you will need to hand in this packet with all data and critical thinking questions completed. You will also need to turn in graphs of the temperature data you just obtained (4 graphs total). You can do your graphs by hand on old-fashioned graph paper, or by using a program such as Excel. It would be best if you could provide one graph that has an overlay of all four graphs. We will discuss in class what components an acceptable graph contains. Critical Thinking Question #1: Does a substance that evaporates more quickly (thus having a greater T) have a higher or lower force of attraction to other molecules in the liquid compared to a substance that evaporates more slowly (smaller T)? Explain. Part 3: Going Against Gravity (Capillary Action) 1) Using the same four liquids, find your four color-coded glass capillaries that should match each of the four liquids. Place the appropriate capillary tube in the vial of liquid your testing, and hold for about 5 seconds. 2) Remove tube and measure how many millimeters of liquid are in the tube. Touch tip of capillary tube to paper towel in order to remove liquid from tube. Use same procedure to get measurements of other three liquids. 3) Use same procedure to test water and hexane in the longer plastic tubes. Water _________ mm Gl a ss C ap il l ar ie s Acetone Ethanol _________ mm _________ mm Hexane _________ mm Pl a st ic C ap il l ar i es Water Hexane _________ mm _________ mm Critical Thinking Question #2: Use your data from the glass capillaries to predict the order of each liquid in terms of its overall polarity. Explain why you ranked them the way you did. Most Polar 1. 2. 3. Least Polar 4. Critical Thinking Question #3: Can you give an explanation for the differences seen between the data from the glass capillaries and those data from the plastic capillaries? Name: _____________________________________________ Assignment #: _____ Due: __________ Part 4: Use the Force to Bend Water (Polarization) 1) Go to the station that contains four burets. 2) Use the fur to rub a plastic rod in order to create a static charge on the rod. 3) Turn on the burets so that all four liquids are flowing into beakers below. Bring the rod horizontal so that it is in front of the liquid streams and slowly move it towards the liquids, looking to see if any of the liquids bend. 4) Try to determine greatest to least deflection (bending) for the four liquids. When you are finished, close the burets and pour the contents of the beakers back into the burets via a funnel at top. Most Deflected 1. 2. 3. Least Deflected 4. Critical Thinking Question #4: If the static electric charge on rod is composed of negative electrons, which chemicals should be deflected more, polar or nonpolar ones? Briefly explain. Part 5: Jedi Blood is Thicker than Water, but is it Thicker than Glycerol? (Viscosity) Go to station that contains the four viscosity tubes. Design your own experiment that will determine the relative polarity of each liquid. Describe your method and provide data (in space below or on next page if you need more room), that ultimately predicts the correct order of polarity for the four liquids in the viscosity tubes. Critical Thinking Question #5: Which should be more viscous, polar or non-polar molecules? Why? Name: _____________________________________________ Assignment #: _____ Due: __________