Chemistry Packet 1: Sections I and II (pg. 3-51)
advertisement
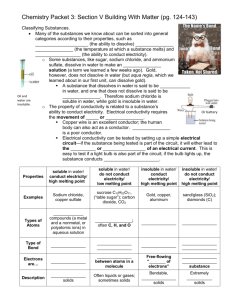
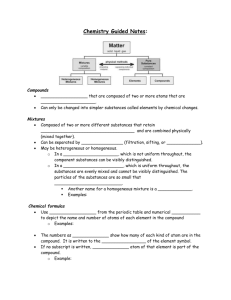
CHEMISTRY SECTION V Chemistry 2014-2015 CLASSIFYING SUBSTANCES Many of the substances we know about can be sorted into general categories according to their properties, such as solubility (the ability to dissolve) melting point (the temperature at which a substance melts) and conductivity (the ability to conduct electricity). Some substances, like sugar, sodium chloride, and ammonium sulfate, dissolve in water to make an aqueous solution (a term we learned a few weeks ago). Gold, however, does not dissolve in water (but aqua regia, which we learned about in our first unit, can dissolve gold). A substance that dissolves in water is said to be soluble in water, and one that does not dissolve is said to be insoluble. Therefore sodium chloride is soluble in water, while gold is insoluble in water. Oil and water are insoluble. The liquid on the left is insoluble in water. The liquid on the right is soluble in water. The property of conductivity is related to a substance’s ability to conduct electricity. Electrical conductivity requires the movement of ions or electrons. Copper wire is an excellent conductor; the human body can also act as a conductor. Rubber is a poor conductor. Electrical conductivity can be tested by setting up a simple electrical circuit—if the substance being tested is part of the circuit, it will either lead to the flow or blockage of an electrical current. This is easy to test if a light bulb is also part of the circuit; if the bulb lights up, the substance conducts electricity. T YPES OF SUBSTANCES We can classify substances into four categories using these properties. Ionic Molecular covalent Metallic Network covalent IONIC Ionic substances are soluble in water, conduct electricity, and have high melting points. Examples: sodium chloride, copper sulfate Types of atoms: ionic compounds (a metal and a nonmetal, or polyatomic ions) in aqueous solution Type of bond: Ionic Electrons are transferred Description: brittle solids Ex. sodium chloride, which can form large “halite” crystals like the one on the right Copper sulfate (left) and cobalt chloride (right). Ionic compounds contain a metal and a nonmetal, and/or polyatomic ions. Many are white crystals, but some are colored. MOLECULAR COVALENT Molecular covalent substances are soluble in water, do not conduct electricity, and have low melting points. Examples: table sugar/sucrose, C 12 H 22 O 11 ; carbon dioxide, CO 2 Types of atoms: nonmetals; often C, H, and O Type of bond: molecular covalent Electrons are shared between atoms in a molecule. Often liquids or gases, sometimes solids In a covalent bond, electrons are shared, like in the bond between two oxygen atoms on the right. Molecular covalent molecules often contain carbon, hydrogen, and/or oxygen. Look at the three molecular covalent compounds below—they contain these elements. METALLIC Metallic substances are insoluble in water, conduct electricity, and have high melting points. Examples: gold, copper, aluminum Types of atoms: metals Type of bond: metallic Free-flowing “sea of electrons” Bendable, malleable, shiny solids Malleable—can be hammered into a thin sheet Metals can often be bent, pressed, hammered, and/or stretched into wires Remember—some of the electrons in metals are freemoving; they are no longer anchored to atoms, but they can move around the entire metal NETWORK COVALENT Network covalent substances are insoluble in water, do not conduct electricity, and have high melting points. Examples: sand/glass (silicon dioxide), diamonds (one form of carbon) Types of atoms: nonmetals Type of bond: network covalent Electrons are shared throughout substance Extremely hard solids Sand, quartz, and glass are all silicon dioxide, SiO2. Note that it contains two nonmetals bonded together in a large network. PACKET 3 EXAMPLE 1: PREDICTING PROPERTIES Predict whether the following substances will dissolve in water, and whether they will conduct electricity. Lead Lead is a metal—look at the properties of metals soluble / insoluble conduct / not conduct Potassium bromide soluble / insoluble “Potassium bromide”—contains a metal and a nonmetal; ionic conduct / not conduct Ionic substances only conduct electricity when they are dissolved in water BONDING Chemists call the attraction that holds atoms together a chemical bond. Several types of bonds exist, and they all involve electrons in some way. We’ve seen that most substances can be divided into four categories based on their physical properties. These categories can be explained by different models of bonding. You can see these in the table on the previous page. Carbon dioxide exists as separate CO2 molecules; silicon dioxide exists as a network of silicon and oxygen. BONDS AND PROPERTIES Some properties are directly related to the type of bonds the atoms in the substances have. Therefore, it is possible to match the bonding with the physical properties observed in different substances. Conduction requires the movement of charged particles. Ionic substances in aqueous solution contain free-moving cations and anions, so they conduct electricity. Metallic substances conduct electricity because their valence electrons are free to move within the solid. Network covalent substances and molecular covalent substances do not contain ions or transfer electrons, so they do not conduct electricity. Melting point depends on the attractive forces between the particles. The higher the melting point, the stronger the attractive forces. Packet 3 Example 2: Identifying Types of Bonds Determine the type of bond in each of the following substances. Then decide the physical properties each substance would have. Substance Magnesium chloride, MgCl2 Rubbing alcohol, C 3H8O Type of Bond Ionic (metal + nonmetal) Molecular covalent (C, H, and O) Soluble in water? Yes Yes Conducts electricity? Electrons are… Yes—when dissolved in water Transferred No Shared between atoms Description Brittle solids Liquid ELECTROPLATING METALS Most metals are dug out of the ground as ionic compounds (ores); in other words, they cannot be found in nature in their pure forms. Through the ages, people have struggled to extract the pure metals from these ores; some are easier to purify than others. This is a piece of gold ore— obviously, it’s not pure gold. Gold and other metals can be extracted from ores using a variety of methods. ALUMINUM Despite being the third most common element in Earth’s crust, aluminum was one of the most difficult metals for scientists to isolate. It was first purified in 1827 by a German chemist named Friedrich Wöhler. Processing aluminum was still difficult and inefficient, making it more expensive per ounce than gold for quite some time. This is bauxite, the ore aluminum can be extracted from. Napoleon III famously let only his favorite guests use his aluminum cutlery, while the rest had to use gold. The Washington Monument was also capped with aluminum which (at the time) was as expensive as silver. It was not until the advent of electrolysis (running an electric current through aluminum ore), that the isolation of aluminum became more efficient, therefore dropping the price of aluminum. ELECTROPLATING Electricity can be used to extract metal from compounds by “giving” electrons back to metal ions, which converts them to neutral metal atoms. This process is called electroplating. Ex. Copper metal can be extracted from a copper sulfate solution by running an electrical current through the solution. ELECTROPLATING WITH GOLD