INTRODUCTION
advertisement

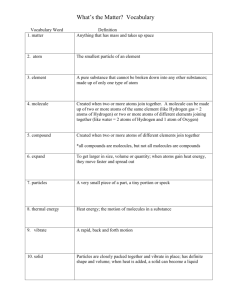
CHAPTER 4. MATTER & ENERGY CHM130 GCC 4.1 Three States of Matter: solid, liquid, and gas Gas: Particles are far apart and are in constant motion. – Gases have no set shape, they assume shape of the container. – Gases have no set volume, it is variable If volume increases, particles move farther apart. If volume decreases, particles move closer together. Liquid: Particles are close together but are free to flow around one another. – Liquids assume shape of the container. – Volume is constant (can’t compress). Solid: Particles are packed tightly together & organized in a rigid pattern; the atoms vibrate in place. – Solids have a definite, fixed shape. – Volume is constant. Cool animations 3 States Of Matter – Scroll down and click on all the states of matter animations and the phase change animation (some may not work which is why there are several options) Ways to Draw the States of Matter physical changes – learn these 6 terms 4.2 Definitions • Element – one type of atom only, can be single atoms (He) or diatomic molecules (O2), cannot be broken down further chemically • Compound – two or more different atoms bonded together, can be broken down chemically into elements • Pure – all particles are the same, cannot be physically separated • Mixture – two or more different particles mixed together, can be physically separated (top picture is pure element, bottom is pure compound) Examples •Mixtures • Metal alloys like 18-K gold, brass • Sand, granite • Tap water • Air which consists of nitrogen, oxygen, and other trace gases. •Pure • Salt (NaCl) is a compound • Diamond (carbon) is an element • Distilled water is a compound • Mercury is an element element compound mixture A = element B = cmpd C = mix D = element E = cmpd F,G = mix H = element For each figure, indicate if it represents an element, a compound, or a mixture 4.3 Elements 1. Each element has a unique name, symbol, and number 2. Capitalize first letter of element name: hydrogen H, carbon C 3. If there’s a 2nd letter it is lower case: helium He, calcium Ca, cobalt Co Careful! CO is carbon monoxide not cobalt Most symbols are from English names: hydrogen H; oxygen O; Helium He Some are from Latin names: lead Pb (plumbum) gold Au (aurum means “golden dawn”) KNOW THE NAMES AND SYMBOLS OF THE FIRST 20 ELEMENTS OF THE PERIODIC TABLE & THE FOLLOWING Ag silver Au gold Pb lead Br Bromine I iodine Hg mercury Periodic Table: You already know many of these Let’s name them! 4.4 nonmetals, semimetals, and metals (Fig. 4.5) 1. Nonmetals (except H) are located on the right side of the stair-step line 2. Semimetals are touching the stair-step line following B (except Al which is metal) 3. Metals are on the left side of the stair-step line Properties of Metals vs. Nonmetals Metals Nonmetals shiny appearance dull appearance malleable, ductile Brittle solids All solids but Hg Many gases density – usually high density – usually low melting point - high melting point low Good conductors of Poor conductors (make heat & electricity better insulators) Semimetals (metalloids) Have properties in between Physical States of the Elements at 25 ˚C and normal atmospheric pressure KNOW THESE Only mercury (Hg) and bromine (Br2) are liquids H2, N2, O2, F2, Cl2, and all Noble gases (Group VIIIA) are gases All other elements are solids Physical States of the elements 04_06.JPG 7 Diatomic elements Diatomic means two atoms bonded together Have no fear of ice cold Beer! H2(g) N2(g) F2(g) O2(g) I2(s) Cl2(g) Br2 (l) Two Diatomic Elements Bromine Br2(l) and Iodine I2(s) I should be able to point at any element and you tell me 1. Solid, liquid, or gas 2. Name (for some of them) 3. Diatomic or not 4. Metal, semimetal, nonmetal Let’s play! I’ll point at several elements… This is how we draw Atoms of an Element 2 or more atoms bonded together = Molecules of an Element if same Molecules of a Compound if different 4.5 Chemical formulas tell us - type of atoms = element symbols - # of those atoms = subscripts (don’t show 1) -But NOT their bonding order Ex: water = H2O 2 H atoms, 1 O atom but water is not bonded H-H-O Ex: How many atoms in potassium nitrate = KNO3 3 O atoms 1 K, ___ 1 N, ___ ___ but it is not bonded K-N-O-O-O Another Example Ex: (NH4)3PO4 What is the total number of atoms? (NH4)3 = 3 NH4’s = 3 ( 1 N + 4 H) = 3N + 12 H TOTAL: 3 N, 12 H, 1 P and 4 O’s = 20 atoms How many atoms of each element are present in Viagra: C22H30N6O4S ? 6 N, ____O, 30 H, ___ 1 22 C, ____ 4 ____ _____S Law of Definite Composition Compounds always contain the same elements in the same proportion by mass. Ex: H2O always contains 11.2 % H and 88.8 % O by mass whether you have a glass full, a swimming pool or an ocean. 4.6 Physical properties - color, odor, taste, texture, melting point, physical state (s, l, or g), density, solubility, conductivity, hardness Chemical properties - describe how a substance reacts or behaves. (explosive, corrosive, toxic, inert, reactive, rusts, oxidizes, decomposes, etc.) 4.7 Physical change: a change that keeps chemical composition the same, the molecules stay the same with the SAME formula. Physical Changes ARE changes in state (s D l D g) Ex: boiling water, melting gold, freezing alcohol, breaking glass, dissolving salt in water, dry ice subliming Note that the H2O molecules remain H2O regardless of whether the sample is a solid, liquid, or gas; changes in physical state are physical changes Review Physical Changes – know these terms! Physical Changes Atoms are always moving, even in solid state. When you heat ice, the water particles gain kinetic energy and move faster. When particles gain enough energy to overcome attractive forces the solid will melt liquid. If we keep heating the liquid, the particles gain more KE & move even faster gaseous state Chemical Changes: a process that changes the chemical composition, the molecules break apart and rearrange. The formula CHANGES. (aka chemical reactions) Starting substance is destroyed and a new substance with different properties is formed. Ex: burning gas Indicators of chemical reaction: These may indicate chemical change • oxidation of matter (burning or rusting) • release of gas bubbles (fizzing) without heating (thus not boiling) • formation of solid (precipitation) • release of heat or light • change in color or odor CHEMICAL REACTIONS REACTANTS PRODUCTS Starting substances are called reactants; New substances formed are called products. Ex: 2 H2 + O 2 2 H2O Ex: Chemical reaction between sodium metal Na(s) and chlorine gas Cl2(g). They produce salt, NaCl, which is a totally different chemical with different formula and properties than the reactants. 4.8 LAW OF CONSERVATION OF MASS Matter or mass cannot be created or destroyed mass of the reactant(s) = mass of the product(s) Two reactants make 4.0 grams of product. If one reactant was 1.5 grams, the other was ________ 2.5 g 4.9 Kinetic Energy - Energy of motion Examples Water flowing over a dam Working out Dancing Burning gasoline Potential Energy- Stored Energy Examples Water behind a dam Gasoline or coal Chemical bonds in food Car at top of roller coaster KE, Temp, and physical state • As kinetic energy increases and molecules move and vibrate faster, the temperature increases ____________. As kinetic energy increases a solid will eventually turn into a liquid ____________. And as the KE increases even more it will eventually turn into a gas ____________. • KE and T are related directly or indirectly? • Which state of matter has lowest KE? solid Highest? gas 4.10 Law of Conservation of Energy Energy cannot be created or destroyed, only converted from one form to another. Ex: When we digest food its stored energy (potential) is converted to kinetic energy to do work. 6 Other Forms of Energy Radiant (light, UV, radiowaves, etc.) Heat Chemical (stored in bonds) Electrical Mechanical Nuclear Chapter 4 Self Test Page 107 •Try # 1, 4-11, 14-16 •Answers in Appendix J