Thermal Energy and Heat
advertisement

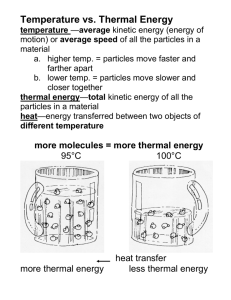
Thermal Energy and Heat Chapter 14 Section 1: Temperature, Thermal Energy, and Heat Temperature • A measure of the average kinetic energy of the individual particles in matter. • The faster the particles move, the more kinetic energy. • We use a thermometer to measure temperature. • 3 Scales: Celsius, Fahrenheit, Kelvin Celsius scale Boiling point of water = 100˚C Freezing point of water = 0˚C Fahrenheit scale Boiling point of water = 212˚F Freezing point of water = 32˚F Kelvin scale Boiling point of water = 373K Freezing point of water = 273K ˚C = K - 273 K = C + 273 formulas • Convert 303K to ˚C • 303K – 273 = 30˚C • Convert 70˚C to K 70˚C + 273 = 343K • absolute zero = 0K or -273 ˚ C • At absolute zero, there is no kinetic energy in the particles / no thermal energy can be removed Thermal Energy and Heat • Thermal Energy depends upon: 1. the number of particles in the object 2. the temperature of the object 3. the arrangement of the particles • Heat is: thermal energy which moves from a warmer object to a less warm object. • Example-ice cube melting in your hand Examine the beakers below. – – – – – Which beaker holds more water molecules? Which beaker has a higher temperature? Which beaker has more kinetic energy? Which beaker has more thermal energy? Which beaker has the greatest mass? Specific Heat • The amount of energy required to raise the temperature of one kilogram of a material by one Kelvin. • Unit for specific heat: J/(kg*K) Formula: change in energy = mass x specific heat x change in temperature • Material with a high specific heat can absorb a lot of thermal energy without a great change in temperature. • Materials with a low specific heat will absorb heat quickly and easily. – Ex: Sand on the beach – Ex: gold (bracelet) – Ex: concrete/pavement/tar – Ex: iron – Ex: copper – Ex: silver Sample Problem • How much heat energy is required to raise the temperature of 5kg of water by 10 Kelvins? • Given information: mass = 5kg change in temp (∆T) = 10K specific heat of water = 4180J/(kg*K) • Unknown: change in energy 3 Steps to Solve This Problem Formula: change in energy = mass x specific heat x ∆T Substitution: energy = 5kg x 4180J/(kg*K) x 10K Answer: 20,900 J Section 2: The Transfer of Heat How is Heat Transferred? • 3 ways that heat can move: –1. Conduction –2. Convection –3. Radiation Conduction • Heat is transferred by the direct contact of particles • Example: the heat energy from the hot soup is transferred by conduction to the spoon—the spoon then becomes warm. Convection • Heat is transferred by the movement of currents within a fluid (liquid or a gas) • Example: boiling water—water is moved by the currents in the fluid • When fluids are heated, the particles move faster and further apart. • Pizza oven • Opening an oven and feeling a blast of hot air • When fluids are heated, they rise upward because they become less dense • Example: hot water/air rises and cool water/air sinks • The rise and fall of these fluids create convection currents (circular motion) Radiation • The transfer of heat energy by electromagnetic waves • Example: fire, sun’s energy • Does not require matter to transfer thermal energy Heat Moves One Way • Heat will always flow from a warmer object to a cooler object. • As thermal energy increases, the temperature of matter absorbing the heat increases. • The temperature of the matter losing the thermal energy is dropping. • Note: there is no such thing as “coldness” Conductors and Insulators • Conductor: material that transfers thermal energy well – metal spoon – most metals • Insulator: material that does not transfer thermal energy well – wood – wool – straw – goose feathers/down comforter – insulation Section 3: Thermal Energy and States of Matter • States of Matter • Solid –Particles are packed tightly together –Particles only vibrate –Retain their shape and volume • Liquid –particles are close together –particles are free to move around –does not have a definite shape –has a definite volume • Gas – particles are moving very fast! – particles are spread very far apart – gases expand to fill available space – lack a fixed shape and volume Changes of State • Change of State: physical change of matter from one state of matter to another (occurs when heat energy is absorbed or released) • Solid-Liquid Changes: • 1. melting: solid changes to a liquid • 2. freezing: liquid changes to a solid • Liquid-Gas Changes: • Vaporization: change from a liquid to a gas (absorbs heat energy) • 1. evaporation: vaporization that takes place at the surface of a liquid • 2. boiling: vaporization that takes place below the surface of a liquid • 3. condensation: a change from a gas to a liquid (cold drink in the summer-loses heat energy) Thermal Expansion • As the thermal energy of matter increases, its particles spread out and the substance expands. • The expansion of matter when it is heated is thermal expansion. • Example: metal grooves on a bridge