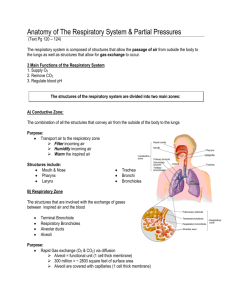
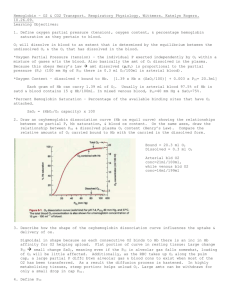
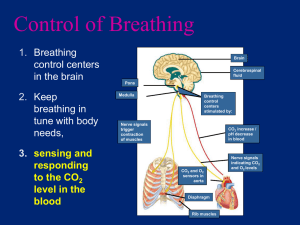
Lecture 10: Respiratory System
advertisement

• Bring balloons and let students blow into balloons • Bring a carbonated beverage • Bring two pieces of transparency film for suction experiment • Bring oximeter Pressure of gases • P = Pressure produced by molecules hitting the walls of a chamber: • P = R (n/V) T – – – – – n = number of particles V=volume (n/V) = concentration T = temperature in Kelvin R = 1.987 cal K-1 mol-1 (gas constant) • If you add another gas to the same compartment, P↑ in proportion to the number of molecules (n). Pressure does not care for the kind of gas. If n is doubled P is doubled. Dalton’s law: the pressure exerted by each gas is independent of pressure exerted by other gases • (this is because gas molecules are so far apart that they do not interfere with each other) • Ptotal = Pgas1 + Pgas2 + Pgas3 + … • these individual pressures are called partial pressures • Example: Pair = P02 + PN2 = 160 + 600 = 760 mm Hg • P02 / Ptotal = 100% · 160/760 = 21% • PN2 / Ptotal = 100% · 600/760 = 79% • PCO2 / Ptotal =100% · 0.3/760= 0.04% 160 mm Hg 600 mm Hg 760 mm Hg Gas dissolved in water • When water is exposed to air containing a particular gas, molecules of the gas will enter water and dissolve in it. • Give me a few examples of a gas dissolved in water? – carbonated beverages: CO2 is dissolved in all carbonated beverages, agitate the bottle and observe CO2 coming out. – Oxygen dissolved in water is used by fish for respiration • In other words, Pgas in air = Pgas in water at equilibrium • Examples: in sea water P02 =? PCO2 =? • Henry’s law: in equilibrium the mass of gas dissolved is proportional to partial pressure of the gas: • [gas] = Pgas · solubility • solubility of O2= 0.03mL / 1L / mmHg • solubility of CO2= 0.6mL / 1L / mmHg • solubility of CO2 in water is 20 fold greater than solubility of O2 • If PO2 =PCO2 then there is 20 time more CO2 molecules than O2 molecules dissolved in water • [O2] on blood at sea level = 160 mm Hg · 0.03mL/1L/mm Hg = 4.8mL / 1L Respiratory system homeostasis plant Breathing feedback control Sensors in medulla in brainstem sensors [CO2]=const controlled variable to a lesser extent O2 and [H+] [H+] (in mM)=24PCO2/[HCO3-] (in nM) • Take a deep breath and … relax all your muscles What happens? • Aren’t you surprised that with all muscles relaxed, the air is being pushed out of you lungs (repeat using your palm to feel the air flow) • Lung elastic recoil is driving expiration, NOT muscles. (muscles stay completely relaxed, i.e. it is a passive process) • Think of the lung as a rubber balloon. Every time you take a breath, the lung wants to recoil back to its un-inflated state. • We say the lung has elastic recoil, just like an elastic balloon. Think of the lung as a rubber balloon The balloon is inflated and glued to the chest wall during inspiration The balloon wants to recoil. To prevent the recoil, you flex the diaphragm Think of the lung as a rubber balloon As soon as you relax the diaphragm, the lungs recoil Expiration volume of fresh air brought in = tidal volume = 500mL • • • • To take the next breath, flex the diaphragm diaphragm pulls on the lungs Inspiration We can also use muscles between ribs to expand the chest by flexing external intercostals or to decrease chest volume by flexing internal intercostals We say: the lung sits in the thoracic cavity. During inspiration ↑ thoracic volume negative pressure (relative to atmospheric 760mm Hg air flows into the lungs What connects lungs to the wall of thorax? filled with water • Nothing! • [Experiment with two pieces of transparency film] • Lungs are connected to the wall of the thorax by suction. First submarines lacked adequate air and power supplies, and were unable to easily lift from the seafloor. Some captains who experimented with landing on the sea floor are still there … water suction prevented their lift from the seafloor. Pneumothorax • Knife, bullet, shrapnel, or broken rib punctures the pleura lung recoils, chest expands • Tension pneumothorax • In 1999 movie Three Kings Mark Wahlenberg’s character “Troy” is shot and suffers from tension pneumothorax • Every marine carries a tension release needle Lung morphology • Bronchi (singular bronchus) divide 2425 times enormous crosssection for easy gas movement • Gas exchange occurs in alveoli • There are 300 million alveoli • diameter of alveolus = 0.3mm • total gas exchange surface area = 75 m2 (=combined area of three classrooms) • Why such a large area? • To facilitate gases exchange Major functions of respiratory system: • O2 exchange and CO2 exchange ambient air: PO2= 160 mm Hg PCO2= 0.3 mm Hg VT is not added to emptiness. It is added to the gas that is left in the lungs (~3L) usually only 1/6 of fresh air is brought into the lungs PO2= 100 mm Hg PCO2= 40 mm Hg PO2= 40 mm Hg PCO2= 46 mm Hg PO2= 100 mm Hg PCO2= 40 mm Hg PO2= 100 mm Hg PCO2= 40 mm Hg PO2= 40 mm Hg PCO2= 46 mm Hg 4.5 – 6 Liters if paralyzed or dead volume will go to FRC 1.5L cannot move it out • During an exercise we need more oxygen we have to bring more fresh air: – We increase the number of breaths per min a little bit – Mostly we ↑ the volume of fresh air that we breathe in with each breath 7Liters How oxygen is carried in blood? • Some oxygen is dissolved in the blood plasma • in 1 liter of oxygenated (arterial) blood – 3 mL of O2 is physically dissolved – 197 mL of O2 is bound to Hb • Hemoglobin (Hb) plays a role of high capacity buffer. It absorbs O2 as a sponge absorbs water O2 0.2µm • In systemic circulation, as O2 dissolved in plasma diffuses into tissue: PO2 ↓ in plasma PO2 ↓ in RBC O2 molecules dissociate from Hb and move into plasma PO2 ↑ in plasma Hemoglobin (Hb) has 4 subunits • Hb is a very special molecule: its affinity to O2 (that is how strong it binds O2) depends on the number of bound O2 molecules • In oxygenated blood (PO2=100mm Hg) Hb binds 4 O2 molecules • As it goes through tissues and releases its 1st O2 molecule, the 2nd O2 molecule is released easier, the 3d even more easier, and the 4th even more easier • This effect occurs because there is positive cooperativity between subunits: as one subunit releases O2, it changes its conformational shape this change in shape reduces other subunits affinity to O2 and so forth Hemoglobin saturation (%) 100% 4 O2 75% 3 O2 =1 molecule of O2 by every Hb molecule 50% 2 O2 25% 1 O2 in moderately active tissue 0 • This positive cooperativity can be represented on a graph that relates the number of O2 molecules held by Hb to PO2 Oximeter • Who has the highest oxygen saturation? • Who has the lowest oxygen saturation? • DPG Lance Armstrong doping case Hemoglobin saturation (%) 100% 4 O2 75% 3 O2 ↑T, ↑[DPG], ↑H+, ↑[CO2] 50% 2 O2 =1 molecule of O2 by every Hb molecule 25% 1 O2 in moderately active tissue 0 • How many O2 molecules are released by every Hb in tissue with ↑T, ↑[2,3-diphosphoglycerate], ↑H+, ↑[CO2] (the thick curve)? • 3 molecules of O2 instead of 1 tissue gets more O2 Notice how smart tissues regulate their O2 supply: • Active muscles – use O2 PO2 in ECF ↓ – release CO2 PCO2 in ECF ↑ – release lactic acid [H+] ↑ – temperature ↑ open precapillary sphincters tissue receives more blood change molecular properties of Hb Hb releases more O2 Precapillary sphincters are relaxed by: Hb affinity for O2 is reduced by: ↓PO2 ↑PCO2 ↑ [H+] ↑ osmolarity ↑ adenosine ↓PO2 ↑PCO2 ↑ [H+] ↑temperature • Conclusions: (1) tissue receives more blood and • (2) each RBC gives up more O2 molecules Myoglobin • • • • Muscles also have their own O2 buffer: Myoglobin has higher affinity to O2 than Hb Therefore at any PO2, O2 will jump from Hb to Mb Myoglobin is analogous to sponge that sacks all O2 from Hb normal PCO2 = 0.3 mm Hg = 0.04% of air by volume PCO2=0.03x760mm Hg=20mmHg PCO2 = 35mm Hg PCO2 = 60mm Hg Examples of CO2 toxicity • In Israel during 1st Gulf war: sealed kitchen + turned on gas for heating two people sleep in the kitchen both die • Because CO2 is heavier than air, in locations where CO2 seeps from the ground (due to sub-surface volcanic or geothermal activity) in high concentrations, CO2 can cause animals and humans to be suffocated: on August 21, 1986, Lake Nyos in Cameroon released of about 300,000 tons of CO2; this cloud rose at nearly 100 km per hour. The gas spilled into a valley and suffocating 3,500 livestock as well as 1,700 people. transport CO2 out of the body 1. 10% of CO2 is dissolved in blood plasma – (compare to O2 : only 1.5% of O2 is dissolved in blood plasma. Solubility of CO2 in water is 20-fold greater than solubility of O2) – 26mL of CO2 is dissolved in 1L of blood (only 3mL of O2) 2. 30% bound to amine group of Lysine and Arginine (amino acids that build Hb). – carbaminoHb – R-NH2 + CO2 <-> R-NH-COO- + H+ 3. 60% as bicarbonate HCO3- – CO2 + H2O <-(slow reaction)-> H2CO3 <-> HCO3- + H+ – the slow reaction is catalyzed by carbonic anhydrase concentrated in RBC • In alveoli CO2 is released into air simply because PCO2 in blood 46 mm Hg is higher than PCO2 in the lungs 40 mm Hg Carbon Monoxide (CO) • CO is a colorless, odorless, and tasteless gas that is slightly less dense than air. • breathe in air with 1% of CO unconscious after 2-3 breathes death in 5 minutes • in the past, motor car exhaust may have contained up to 25% CO. Newer cars have catalytic converters which eliminate 99% of CO produced. However even cars with catalytic converters produce enough CO to kill if the car is left idling in enclosed space. • CO is produced by burning firewood firemen sometimes suffer brain damage; occipital lobe is first to die… • What is going on? • CO has higher affinity to Hb than O2. CO binds 240 times tighter to Hb CO essentially displaces O2. Once CO is bound to Hb, Hb cannot carry any O2. Arterial blood still has PO2=100mm Hg, but total [O2]=1.5% of normal. That 3mL of O2 is still dissolved in oxygenated blood, but the Hb buffer has no O2. When blood travels to tissue, this dissolved O2 quickly diffused to tissue and there is no O2 to replace plasma dissolved oxygen. • CO is produced in the process of Hb recycling 1% of Hb is usually bound to internally produced Hb. In smokers up to 10% of Hb is bound to CO. Sensors • Two sets of chemoreceptors: 1. central (in medulla, close to medulla respiratory control center) 2. peripheral (sinuses of carotid arteries and the arch of aorta – these are physically close but distinct from arterial baroreceptors) midbrain • 3 substances to control: change: CO2 change: O2 – CO2 – O2 – H+ • Which is the most important for setting ventilation? • Experiments: – fix O2, vary CO2 without telling to a subject, measure ventilation – fix CO2, vary O2, measure ventilation • CO2 is most important for setting ventilation rate • makes sense CO2 reflects activity level: double activity level double CO2 production double ventilation • It looks like in a lot of cases this mechanism does not kick in before humans die. There are plenty of stories of humans walking into deoxygenated chamber and dies without feeling of danger pH • Recall: 60% of CO2 is transported as bicarbonate HCO3- – CO2 + H2O <-(slow reaction)-> H2CO3 <-> HCO3- + H+ • Thus PCO2 and [H+] in blood are linked: • [H+] (in mM)=24PCO2/[HCO3-] (in nM) • Increased PCO2 immediately increases H+ and vise versa • In fact chemoreceptors (both central and peripheral) are sensitive to [H+] • (the peripheral receptors, but not central receptors are sensitive to PO2) • Effects of increased PCO2 (hence increase [H+]) and decreased O2 are independent inputs to medulla. Their combined effect is synergistic: it is considerably greater than the sum of the individual responses. • CO2 is most important for setting ventilation rate. Acts on both peripheral and central receptors via [H+]. • Respiratory muscles are skeletal muscles need input from nerves • In medulla there are two groups of neurons working as pacemaker that can be regulated by chemoreceptors • The biggest respiratory muscle is diaphragm • Inspiration diaphragm is stimulated by motor nerves in the phrenic nerve (leaving spinal cord on 3,4,5 cervical vertebra). • If you need to increase ventilation double firing in motor neuron • Expiration ? • Expiration is passive lung elastic recoil pushes air out of lungs. • Emphysema: lungs loose elastic recoil lungs stay inflated. • Medulla control of PCO2 and PO2 is reflexive • These reflexes can be overridden: for example, we interrupt breathing to talk and to sing. • On the other hand, reflex is a reflex: run for a mile, then try to give a speech you cannot because respiration is overriding the attempt to speak. Summary plant CNS voluntary input feedback control Breathing Sensors in medulla in brainstem [CO2]=const controlled variable to a lesser extent O2 and [H+] [H+] (in mM)=24PCO2/[HCO3] (in nM) sensors Functions of respiratory system: • oxygen exchange • CO2 exchange • Regulates H+ (blood pH) • Forms speech sounds • Defends against microbes (immune function) • Traps and dissolves blood clots Asthma • Affects 7% population in USA • Causes 4,000 deaths a year in USA • Asthma attack is relieved by albuterol (quick-acting adrenergic receptor agonist) that relaxes smooth muscle on bronchi • Long-term asthma treatment: avoid exposure to allergens; reduce bronchi inflammation with corticosteroids • Practicing breathing exercise • consider discussing both cardivascular and respiratory HW • Expiration is passive lung elastic recoil pushes air out of lungs. • Emphysema: lungs loose elastic recoil lungs stay inflated.