File
advertisement

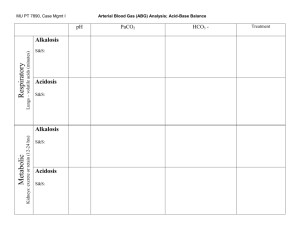
pH is a measurement of the acidity of the blood, reflecting the number of hydrogen ions present. Lower numbers mean more acidity; higher number mean more alkalinity. pH is Elevated (more alkaline, higher pH) with: Hyperventilation Anxiety, pain Anemia Shock Some degrees of Pulmonary disease Some degrees of Congestive heart failure Myocardial infarction Hypokalemia (decreased potassium) Gastric suctioning or vomiting Antacid administration Aspirin intoxication pH is Decreased (more acid, lower pH) with: Strenuous physical exercise Obesity Starvation Diarrhea Ventilatory failure More severe degrees of Pulmonary Disease More severe degrees of Congestive Heart Failure Pulmonary edema Cardiac arrest Renal failure Lactic acidosis Ketoacidosis in diabetes pCO2 (Partial Pressure of Carbon Dioxide) reflects the the amount of carbon dioxide gas dissolved in the blood. Indirectly, the pCO2 reflects the exchange of this gas through the lungs to the outside air. Two factors each have a significant impact on the pCO2. The first is how rapidly and deeply the individual is breathing: Someone who is hyperventilating will "blow off" more CO2, leading to lower pCO2 levels Someone who is holding their breath will retain CO2, leading to increased pCO2 levels The second is the lungs capacity for freely exchanging CO2 across the alveolar membrane: With pulmonary edema, there is an extra layer of fluid in the alveoli that interferes with the lungs' ability to get rid of CO2. This leads to a rise in pCO2. With an acute asthmatic attack, even though the alveoli are functioning normally, there may be enough upper and middle airway obstruction to block alveolar ventilation, leading to CO2 retention. Increased pCO2 is caused by: Pulmonary edema Obstructive lung disease Decreased pCO2 is caused by: Hyperventilation Hypoxia Anxiety Pregnancy Pulmonary Embolism (This leads to hyperventilation, a more important consideration than the embolized/infarcted areas of the lung that do not function properly. In cases of massive pulmonary embolism, the infarcted or non-functioning areas of the lung assume greater significance and the pCO2 may increase.) PO2 (Partial Pressure of Oxygen) reflects the amount of oxygen gas dissolved in the blood. It primarily measures the effectiveness of the lungs in pulling oxygen into the blood stream from the atmosphere. Elevated pO2 levels are associated with: Increased oxygen levels in the inhaled air Polycythemia Decreased PO2 levels are associated with: Decreased oxygen levels in the inhaled air Anemia Heart decompensation Chronic obstructive pulmonary disease Restrictive pulmonary disease Hypoventilation CO2 Content is a measurement of all the CO2 in the blood. Most of this is in the form of bicarbonate (HCO3), controlled by the kidney. A small amount (5%) of the CO2 is dissolved in the blood, and in the form of soluble carbonic acid (H2CO3). For this reason, changes in CO2 content generally reflect such metabolic issues as renal function and unusual losses (diarrhea). Respiratory disease can ultimately effect CO2 content, but only slightly and only if prolonged. Elevated CO2 levels are seen in: Severe vomiting Use of mercurial diuretics COPD Aldosteronism Decreased CO2 levels are seen in: Renal failure or dysfunction Severe diarrhea Starvation Diabetic Acidosis Chlorthiazide diuretic use Base Excess or Base Deficit Whenever there is an accumulation of metabolically-produced acids, the body attempts to neutralize those acids to maintain a constant acid-base balance. This neutralizing is achieved by using up various "buffering" compounds in the blood stream, to bind the acids, disallowing them from contributing to more acidity. About half of these buffering compounds come from HCO3, and the other half from plasma and red blood cell proteins and phosphates. The words "base deficit" and "base excess" are equivalent and are generally used interchangeably. The only difference is that base deficit is expressed as a positive number and base excess is expressed as a negative number. A "Base Deficit" of 10 means that 10 mEqu/L of buffer has been used up to neutralize metabolic acids (like lactic acid). Another way to say the same thing would be the "Base Excess is minus 10." More Negative Values of Base Excess may Indicate: Lactic Acidosis Ketoacidosis Ingestion of acids Cardiopulmonary collapse Shock More Positive Values of Base Excess may Indicate: Loss of buffer base Hemorrhage Diarrhea Ingestion of alkali Oxygen Saturation (SO2) measures the percent of hemoglobin which is fully combined with oxygen. While this measurement can be obtained from an arterial or venous blood sample, it's major attractive feature is that it can be obtained non-invasively and continuously through the use of a "pulseoximeter." Normally, oxygen saturation on room air is in excess of 95%. With deep or rapid breathing, this can be increased to 98-99%. While breathing oxygen-enriched air (40% - 100%), the oxygen saturation can be pushed to 100%. Oxygen Saturation will fall if: Inspired oxygen levels are diminished, such as at increased altitudes. Upper or middle airway obstruction exists (such as during an acute asthmatic attack) Significant alveolar lung disease exists, interfering with the free flow of oxygen across the alveolar membrane. Oxygen Saturation will rise if: Deep or rapid breathing occurs Inspired oxygen levels are increased, such as breathing from a 100% oxygen source