Identifying and Naming Acids and Bases
advertisement

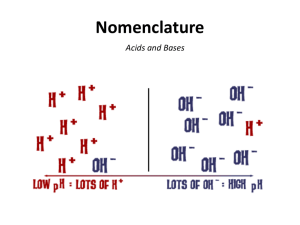
Acids and Bases Acids • • • • • • • Sour taste Conduct electricity React with metals to produce H2(g) React with carbonates to produce CO2(g) React with bases to produce water and a salt Turn blue litmus red Are formed when non-metal oxides react with water. e.g. CO2 + H2O H2CO3(aq) Identifying Acids • Usually the name of an acid ends with the word “acid” • The chemical formula starts with the symbol for hydrogen (H) and ends in (aq) (e.g. HCl(aq)) or • The chemical formula ends with COOH (the symbol for the carboxyl polyatomic ion) and ends in (aq) (e.g. CH3COOH(aq)) Naming Acids • We will use two general rules for naming acids in this course: I. Binary Acids: Starts with H and has only one other non-metal: 1. Start with the prefix “hydro” 2. Change the ending of the non-metal to “ic” and add the word acid. e.g. HCl(aq) - hydrochloric acid HF(aq) - hydrofluoric acid HBr(aq) - hydrobromic acid HI(aq) - hydroiodic acid H2S(aq) - hydrosulfuric acid II. Oxy-Acids: Starts with H and contains a polyatomic ion that ends in “ate”. 1. Start with the name of the element in the polyatomic ion that is not oxygen. 2. Change the element’s ending to “ic” and add the word “acid” e.g. H2SO4(aq) - sulfuric acid H3PO4(aq) - phosphoric acid HNO3(aq) - nitric acid H2CO3(aq) - carbonic acid HClO3(aq) - chloric acid Common Acids HCl(aq) – hydrochloric acid (muriatic acid, stomach acid) H2SO4(aq) – sulfuric acid (battery acid) HCOOH(aq) – methanoic acid (formic acid) CH3COOH(aq) – ethanoic acid (acetic acid) Bases • • • • • • Bitter taste Conduct electricity Feel slippery React with acids to produce water and a salt Turn red litmus blue Are formed when metal oxides react with water. e.g. NaO + H2O NaOH(aq) Identifying Bases • Usually the name begins with the name of a metal ion or ammonium and ends with the word “hydroxide” or “carbonate” • The chemical formula starts with a metallic ion or with the ammonium ion (NH4+) AND • The chemical formula ends with OH- or CO32- Naming Bases • The name of a base can be determined by its chemical formula. 1. Write the name of the positively charged metal ion or positively charged polyatomic ion that is at the beginning of the chemical formula. 2. Add the word “hydroxide” or “carbonate” at the end (depending on which is there) e.g. KOH(aq) - potassium hydroxide NH4OH(aq) - ammonium hydroxide CaCO3(aq) - calcium carbonate Common Bases • NaOH(aq) – sodium hydroxide (caustic soda, lye) • NH4OH(aq) – ammonium hydroxide (ammonia solution) • Ca(OH)2(aq) – calcium hydroxide (slaked/hydrated lime) • Mg(OH)2(aq) – magnesium hydroxide (milk of magnesia) • NaHCO3(aq) – sodium bicarbonate (baking soda) http://gestalta.net/view.php?video=sXybLXZU9EQ&feature=youtube_gdata_player&title=1977+Arm+%26+Hammer+%22Onion+Power%22+Commercial Acids and Bases in the Body Neutralization Reactions • A neutralization reaction occurs when an acid and a base are reacted together. • The two products are both NEUTRAL meaning they are not acidic or basic. acid + base water + salt both neutral e.g. HCl(aq) + NaOH(aq) H2O(l) + NaCl(aq) Strong vs Weak • A strong acid or base breaks into its ions more easily than a weak acid or base does. • For example: Strong: HCl H+ + Cl(almost all of the HCl molecules break up into H+ and Cl- ions) Weak: HC2H3O2 H+ + C2H3O2(most of the HC2H3O2 DO NOT break into ions so there are only a small amount of H+ ions) Concentrated vs Dilute • Acids and bases are solutions, which means they are dissolved in water. • If there is very little water added, the acid or base will be concentrated. • If there is a lot of water added, the acid or base will be dilute. • For example, a concentrated acid will have more H+ ions and therefore have a lower pH than a dilute acid.