Isopentyl Acetate Synthesis: Banana Oil Lab
advertisement

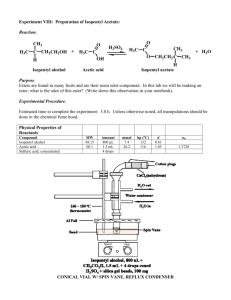
Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Resources Pavia: 697 p. 84 – 91; 686 – 688; 693 – Lab Website: http://classweb.gmu.edu/chemlab Schornick: 3/12/2016 http://classweb.gmu.edu/ jschorni/chem318 1 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Overview Synthesis – Acid (H2SO4) catalyzed Fischer Esterification reaction of a Carboxylic Acid (Acetic Acid) with the Hydroxyl group of an Alcohol (Isopentyl [Amyl] Alcohol) This is a Condensation reaction where the molecules become joined through the intermolecular elimination of water 3/12/2016 Refluxing - Reagents will be Refluxed for about 60 minutes. Liquid/Liquid Extraction - The product will be washed several times with Water, Sodium Bicarbonate, Sodium Chloride Dry Sample Refractive Index (with temperature correction) Infrared Spectrum 2 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 The Laboratory Report: Synthesis Experiment Mass, Moles, Molar Ratio, Limiting Reagent, Theoretical Yield Procedures Title – Concise: Simple Distillation, Dry Sample, IR Spectrum, etc. Materials & Equipment (2 Columns in list (bullet) form) Note: include all reagents & principal equipment used, including details of distillation setup Description: Use list (bullet) form Concise, but complete description Use your own words – Don’t copy book!! 3 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 The Laboratory Report: (Con’t) Results – Neat, logically designed template to present results Summary – experimental results, including Theoretical Yield results, in paragraph form Analysis & Conclusions Limiting reagent Discuss reaction mechanism in the context of your experimental results Verification of product 4 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 5 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Reagents Acetic Acid Mol Wgt - 60.05 g/mole Density - 1.049 g/mL Boiling Point - 117.9oC % Acid - 100% Isopentyl (amyl) Alcohol Mol Wgt - 88.15 g/mole Density - 0.809 g/mL Boiling Point - 130.0oC Sulfuric Acid Mol Wgt - 98.08 g/mole Density - 1.84 g/mL % Acid - 96.5 % Molarity - 36 moles/Liter 3/12/2016 Product Isopentyl (amyl) Acetate Mol Wgt - 130.19 g/mole Density - 0.876 Boiling Pnt - 142oC Ref. Index - 1.4040 6 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Heating Under Reflux The reaction mixture is heated in a “reflux apparatus” consisting of a 25 or 50 mL round bottom flask attached to a vertical watercooled condenser set in a heating mantle. Liquid/Liquid Extraction 3/12/2016 7 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Elements of the Experiment 3/12/2016 Equilibrium Reaction equilibrium does not favor formation of the ester Therefore, an excess of one of the reagents (Acetic Acid – Cheap!!!) is used to force the equilibrium to product side, i.e., to the right 8 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Elements of the Experiment (Con’t) 3/12/2016 Liquid/Liquid Extraction The reaction mixture is mixed with Distilled Water and placed in a separatory funnel where the phases separate into two layers – Aqueous (higher density) layer on bottom and the Organic layer on top. Separate the Aqueous phase into a waste beaker Extract Organic phase twice with 5% Sodium Bicarbonate to remove excess acid. Discard Aqueous phase into waste beaker Extract Organic phase twice with Saturated Sodium Chloride to remove excess water. Discard Aqueous phase into waster beaker 9 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 Elements of the Experiment (Con’t) Dry Product – Dry crude ester (organic layer) with Anhydrous Sodium Sulfate (less than 1 gram) Mass of Product Percent (%) Yield Refractive Index corrected for temperature Infrared (IR) Spectrum 10 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 Procedure Measure out ~10 mL of Glacial Acetic Acid to the nearest 0.1 mL and compute the mass from the volume and density Weigh the Isopentyl Alcohol in the vial to the nearest 0.001 g Mix the reagents in a Beaker, swirling gently to ensure complete solution If necessary, heat gently to help dissolution of reactants Carefully add 1.0 mL concentrated Sulfuric Acid to the mixture mixing (swirling) quickly 11 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 Procedure (Con’t) Set up a “Reflux” Apparatus using a 50 mL round bottom flask Add a “Corundum” or “Teflon” (not calcium carbonate) boiling chip and heat the mixture under “Gentle” reflux for 50 – 60 minutes Cool the mixture to Room Temperature Liquid/Liquid Extraction Pretest the Separatory Funnel to ensure that the stopper and the stopcock assembly do not leak Add about 50 mL distilled water to the funnel Shake vigorously for about 10 seconds Adjust stopper or stopcock as necessary and repeat Drain water from funnel 12 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Procedure 3/12/2016 Liquid/Liquid Extraction (con’t) Transfer the reaction mixture to the Separatory Funnel using a short neck stem funnel Note: Do not transfer boiling chip to funnel Rinse reaction vessel with 10 mL Distilled Water and add to mixture in separatory funnel Mix phases by shaking and venting Remove the lower aqueous layer and put it aside for later disposal 13 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Procedure (Con’t) Liquid/Liquid Extraction (Con’t) Extract the upper organic layer with 10 mL aqueous Sodium Bicarbonate (NaHCO3) to remove excess Acid. Note: The reaction between any residual acid and Sodium Bicarbonate produces CO2 gas and could be under considerable pressure in the separatory funnel. Hold the stopper firmly, invert the funnel, and release the stopcock very slowly so the venting of the gas is controlled 3/12/2016 14 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Procedure (Con’t) 3/12/2016 Liquid/Liquid Extreaction (Con’t) Remove the lower aqueous layer and add to previous waste aqueous solution Repeat the NaHCO3 extraction a second time Perform a third extraction of the upper organic layer in the Separatory Funnel with 10 mL of saturated Sodium Chloride to remove excess water Remove the lower aqueous layer and add to previous waste aqueous solution 15 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) Procedure (Con’t) Liquid/Liquid Extraction (Con’t) Repeat the Sodium Chloride extraction a second time Transfer the organic layer containing the crude ester to a small, clean, Erlenmeyer flask Instructor will add 1.0 g of Anhydrous Sodium Sulfate Note: If mixture does not appear to dry (drying agent will clump), add a little more Sodium Sulfate 3/12/2016 16 Synthesis of Isopentyl (Amyl) Acetate Ester (Banana Oil) 3/12/2016 Procedure (Con’t) Allow the mixture to settle Decant the dried sample into a pre-weighed 100 mL beaker, leaving all solid material in the flask Reweigh the beaker and determine the mass of product Determine percentage yield Measure the Refractive Index and correct for temperature Obtain the IR Spectrum 17