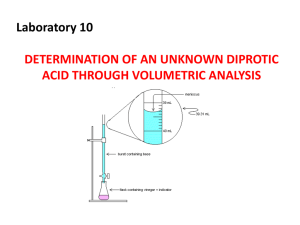
Honors Acid Base - snarrenberg-chem
advertisement

Good mornin’ Lot’s of things to pick up today: Quiz Scantron Quiz version that you took Lab Sheet Final Study Guide Dry Erase Board Marker 10 minutes to look over missed quiz questions & answer the Catalyst pH Test Tube • • • • • [H+] (mol/L) pH 1 0.01 <4 2 0.001 <4 3 0.0001 4 4 0.00001 5 5 0.000001 6 How does the molarity of H+ change? How does the pH change? Why are the pH’s for test tubes 1 and 2 indicated as “<4”? What is an Arrhenius acid? What is an Arrhenius base? What is a Bronsted-Loewry acid? What is a Bronsted-Loewry base? Example Brønsted Acids and Bases: NH3 + HOH + NH4 + OH Here, H2O acts as a Brønsted acid by donating a proton to NH3 which acts as a Brønsted base. Conjugate base and conjugate acid? pH pH is a scale used to reflect the concentration of H+ ions! Acids and bases are aqueous solutions Water can act as an acid and a base: HOH <--> H+ + OH- Why is water neutral? Acids dissociate and increase the number of protons (H+) in solution Bases dissociate and increase the number of hydroxide (OH-) in solution either directly (Arrhenius) or indirectly (Bronsted-Loewry) Neutralization Reaction Remember that this is just an acid base reaction What would a product of a neutralization reaction be? Just like the stoichiometry you’ve been doing, in lab we can use this fact to calculate the molarity of solution whose concentration is unknown Titration standard solution Definition ◦ Analytical method in which a standard solution is used to determine the concentration of an unknown solution. unknown solution Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Buret stopcock Erlenmeyer flask Titration Vocabulary Titrant ◦ The substance added to the analyte in a titration (a standard solution) Analyte ◦ The substance being analyzed Equivalence point ◦ The point in a titration at which the quantity of titrant is exactly sufficient for stoichiometric reaction with the analyte. If the concentration of the titrant is Acid-Base Titration known, then the unknown concentration of the analyte can be determined. Titrant Analyte Buret Reading Why do chemists use titrations?? Quantitative analysis — used to determine the amounts or concentrations of substances present in a sample by using a combination of chemical reactions and stoichiometric calculations Acidic, basic, or neutral?? The “perfect pink” for a titration with phenolphthalein Indicator changes color to indicate pH change pH Example… 7 phenolphthalein is colorless in acid and pink in basic solution point at which exactly enough reactant pink has been added for Endpoint = the solution to be neutralized and no Volume base added more Equivalence point (endpoint) Point at which equal amounts of H3O+ and OH- are present in solution. Determined by… indicator color change dramatic change in pH Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Titration moles H3 + O = moles Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem OH White Board Review! Solubility Molarity Dilutions Solutions Stoichiometry Acid and Base Problem #1 What mass of (NH4)2SO4 is required to make 1.25 L of a 0.250 M solution of NH4+? Answer: 20.6 g (mm=132.14g/mol) Problem #2 If 25 g of KCl is added to 50 g of water at 40°C, the solution would be: 1) Unsaturated 2) Saturated 3) Supersaturated Problem #3 Calculate the molarity of a solution prepared by dissolving 4.1 g of solid KBr in enough water to make 1.10 L of solution Answer: 0.031 M (mm=119.02 g/mol) Problem #4 One way to determine the amount of chloride ion in a a water sample is to titrate the sample of standard AgNO3 solution to produce solid AgCl. Ag+(aq) + Cl-(aq) AgCl(s) If a 25.0 mL water sample requires 27.2 mL of 0.104 M AgNO3 in such a titration, what is the concentration of Cl- in the sample? Answer: 0.113 M Problem #5 What volume of of a a 5.00M Ca(NO3)2 solution is needed to prepare 465 mL of a 0.250 M Ca(NO3)2 solution? Answer: 23.3 mL Problem #6 Calculate the mass of sodium iodide that must be added to 425.0 mL of a 0.100 M lead (II) nitrate solution to precipitate all of the lead (II) ions as lead (II) iodide. Answer: 12.7 g NaI