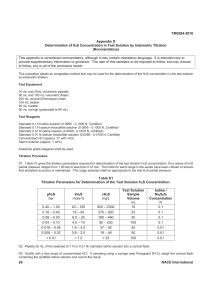
POST-LABORATORY REPORT – EXPERIMENT NO. 5 Titrimetric Analysis SECTION: ____________ GROUP NO.: ______ NAME WITH SIGNATURE: DATE PERFORMED: ____________ DATE SUBMITTED: _____________ INSTRUCTORS: ____________________ 1. Draw a simple titration set-up and label its parts. 2. Briefly explain why each burette must be rinsed with the solution to be used. 3. What is molarity of a solution of KOH, 25.0 mL of which requires 29.2 mL of 0.200 M H2SO4 for reaction? 4. Draw the titration curve for a strong acid (titrant) and a strong base (analyte) titration. Kindly show and label the x- and y- axes, and the equivalence point.