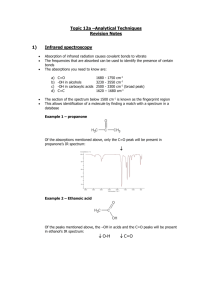
infra red spectroscopy

INFRA RED
SPECTROSCOPY
A guide for A level students
KNOCKHARDY PUBLISHING
KNOCKHARDY PUBLISHING
INFRA RED SPECTROSCOPY
INTRODUCTION
This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards.
Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available.
Accompanying notes on this, and the full range of AS and A2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at...
www.knockhardy.co.uk/sci.htm
Navigation is achieved by...
either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard
INFRA RED SPECTROSCOPY
CONTENTS
• Vibrations of bonds in molecules
• The Infra Red spectrophotometer
• Interpretation of IR spectra
• Characteristic absorption frequencies
INFRA RED SPECTROSCOPY
Before you start it would be helpful to…
• know the names and structures of organic functional groups
INFRA RED SPECTROSCOPY
Different covalent bonds have different strengths due to the masses of different atoms at either end of the bond.
As a result, the bonds vibrate at different frequencies
The frequency of vibration can be found by detecting when the molecules absorb electro-magnetic radiation.
Various types of vibration are possible.
INFRA RED SPECTROSCOPY
Different covalent bonds have different strengths due to the masses of different atoms at either end of the bond.
As a result, the bonds vibrate at different frequencies
The frequency of vibration can be found by detecting when the molecules absorb electro-magnetic radiation.
Various types of vibration are possible.
Examples include... STRETCHING and BENDING
SYMMETRIC
STRETCHING
BENDING ASYMMETRIC
STRETCH
BENDING AND STRETCHING IN WATER MOLECULES
SYMMETRIC STRETCHING
BENDING AND STRETCHING IN WATER MOLECULES
ASYMMETRIC STRETCHING
BENDING AND STRETCHING IN WATER MOLECULES
BENDING
The Infra-red Spectrophotometer
• a beam of infra red radiation is passed through the sample
• a similar beam is passed through the reference cell
• the frequency of radiation is varied
• bonds vibrating with a similar frequency absorb the radiation
• the amount of radiation absorbed by the sample is compared with the reference
• the results are collected, stored and plotted
The Infra-red Spectrophotometer
A bond will absorb radiation of a frequency similar to its vibration(s) normal vibration vibration having absorbed energy
IDENTIFICATION OF
PARTICULAR BONDS
IN A MOLECULE
INFRA RED SPECTRA USES
The presence of bonds such as O-H and C=O within a molecule can be confirmed because they have characteristic peaks in identifiable parts of the spectrum.
IDENTIFICATION OF
PARTICULAR BONDS
IN A MOLECULE
INFRA RED SPECTRA USES
The presence of bonds such as O-H and C=O within a molecule can be confirmed because they have characteristic peaks in identifiable parts of the spectrum.
IDENTIFICATION OF
COMPOUNDS BY DIRECT
COMPARISON OF SPECTRA
The only way to completely identify a compound using IR is to compare its spectrum with a known sample.
The part of the spectrum known as the ‘Fingerprint Region’ is unique to each compound.
INFRA RED SPECTRA INTERPRETATION
Infra-red spectra are complex due to the many different vibrations taking place in each molecule.
INFRA RED SPECTRA INTERPRETATION
Infra-red spectra are complex due to the many different vibrations taking place in each molecule.
Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory.
INFRA RED SPECTRA INTERPRETATION
Infra-red spectra are complex due to the many different vibrations taking place in each molecule.
Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory.
The technique is useful when used in conjunction with other methods nuclear magnetic resonance spectroscopy and mass spectroscopy.
INFRA RED SPECTRA INTERPRETATION
Infra-red spectra are complex due to the many different vibrations taking place in each molecule.
Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory.
The technique is useful when used in conjunction with other methods nuclear magnetic resonance spectroscopy and mass spectroscopy.
Peak position depends on bond strength masses of the atoms joined by the bond strong bonds and light atoms absorb at lower wavenumbers weak bonds and heavy atoms absorb at high wavenumbers
INFRA RED SPECTRA INTERPRETATION
Vertical axis Absorbance
Horizontal axis Frequency
Wavelength the stronger the absorbance the larger the peak wavenumber (waves per centimetre) / cm -1 microns (m); 1 micron = 1000 nanometres
FINGERPRINT REGION
• organic molecules have a lot of C-C and C-H bonds within their structure
• spectra obtained will have peaks in the 1400 cm -1 to 800 cm -1 range
• this is referred to as the “fingerprint” region
• the pattern obtained is characteristic of a particular compound the frequency of any absorption is also affected by adjoining atoms or groups.
IR SPECTRUM OF A CARBONYL COMPOUND
• carbonyl compounds show a sharp, strong absorption between 1700 and 1760 cm -1
• this is due to the presence of the C=O bond
IR SPECTRUM OF AN ALCOHOL
• alcohols show a broad absorption between 3200 and 3600 cm -1
• this is due to the presence of the O-H bond
IR SPECTRUM OF A CARBOXYLIC ACID
• carboxylic acids show a broad absorption between 3200 and 3600 cm -1
• this is due to the presence of the O-H bond
• they also show a strong absorption around 1700 cm -1
• this is due to the presence of the C=O bond
IR SPECTRUM OF AN ESTER
• esters show a strong absorption between 1750 cm -1 and 1730 cm -1
• this is due to the presence of the C=O bond
WHAT IS IT!
One can tell the difference between alcohols, aldehydes and carboxylic acids by comparison of their spectra.
O-H STRETCH
ALCOHOL
C=O STRETCH
ALDEHYDE
O-H STRETCH
AND
C=O STRETCH
CARBOXYLIC
ACID
CHARACTERISTIC FREQUENCIES
N-H C
N
O-H
C-H
C=O
Aromatic C-C
C=C
C-O
C-C alkanes
C-Cl
Bond
C-H
C-C
C=C
CHARACTERISTIC ABSORPTION FREQUENCIES
Class of compound
Alkane
Alkane
Alkene
Range / cm -1
2965 - 2850
1200 - 700
1680 - 1620
Intensity strong weak variable
C=O
C-O
Ketone
Aldehyde
Carboxylic acid
Ester
Amide
Alcohol, ester, acid, ether
1725 - 1705
1740 - 1720
1725 - 1700
1750 - 1730
1700 - 1630
1300 - 1000 strong strong strong strong strong strong
O-H
N-H
C
N
C-X
Alcohol (monomer)
Alcohol (H-bonded)
Carboxylic acid (H-bonded)
Amine, Amide
Nitrile
Chloride
Bromide
Iodide
3650 - 3590
3420 - 3200
3300 - 3250
3500 (approx)
2260 - 2240
800 - 600
600 - 500
500 (approx) variable, sharp strong, broad variable, broad medium medium strong strong strong
REVISION CHECK
What should you be able to do?
Understand the origin of IR spectra
Identify peaks associated with O-H and C=O bonds
Contrast the spectra of alcohols, carbonyls and carboxylic acids
CAN YOU DO ALL OF THESE?
You need to go over the relevant topic(s) again
Click on the button to return to the menu
WELL DONE!
Try some past paper questions
INFRA RED
SPECTROSCOPY
THE END
© 2005 JONATHAN HOPTON & KNOCKHARDY PUBLISHING