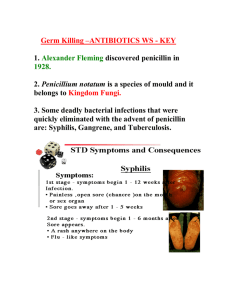
Drugs and Medicine student slide show 2013
advertisement

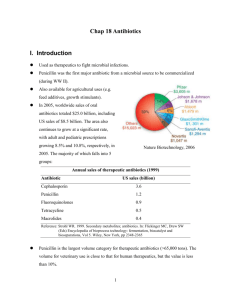
Option D – Medicine and Drugs IB Chemistry Outline of this option Pharmaceutical products ( definitions, categories, placebo effect, body's defenses, research, Thalidomide, administration of drugs, toxicity, tolerance) Antacids Analgesics ( mild - aspirin, acetaminophen, ibuprofen strong (opiates) - morphine, codeine, heroin) Depressants ( tranquilizers - alcohol, "other") Stimulants (adrenaline (epinephrine) amphetamines - ecstasy, pheylethylamine, dopamine, "speed") Antibacterials (penicillin) Antivirals (AIDS) What is a medicine or drug? · A substance that alters one or more of the following: ·Incoming sensory sensations ·Mood or emotions ·Physiological state ·Consciousness ·Activity level ·Co-ordination D.1 Pharmaceutical Products A drug or medicine is any chemical which: Alters sensory sensations Alters mood or emotions Alters physiological state (consciousness, activity level, or coordination) Drugs: chemicals that affect how the body works. Used to describe substances both legal and illegal. Medicines: substances that improve health. Can be natural or synthetic. The beneficial effects of medicines are known as the therapeutic effect. Categories of medicines ·Infection fighters ·Antiseptics ·Antibiotics ·Antivirals ·Affecting metabolism ·Hormones ·Vitamins ·Affecting central nervous system ·Stimulants ·Depressants ·Analgesics ·Anaesthetics Placebo effect: ·A pharmacologically inert substance (often a sugar pill) produces a significant reaction because the patient expects, desires, or was told it would happen ·Used as a control in clinical trials ·Highlights the body’s natural healing powers and power of suggestion · Body’s Lines of Defense First line defense: ·Skin, mucous membranes, closures and secretions (lips, eyelids, ear wax, etc.) ·Second line defense: ·White blood cells, blood clotting, inflammatory response ·Third line defense: ·Antibodies, memory cells · Research on new products 1. Tests on animals ·Dose, side effects ·2. Clinical trial (phase 1) ·Safety, dose range ·3. Clinical trial (phase 2) ·Response, investigator bias, statistics ·4. Clinical trial (phase 3) ·Extended evaluation · D.1.2 Research and Development: ·Development of a new drug is a very costly, lengthy process controlled by the government: · ·In 1970, 3620 drugs were tested. 16 came on the market at an average cost of $20 million ·Only 1 in 2000 drugs eventually make it to the market ·Phase I: Initial clinical trials on volunteers after the drug has proven safe when given to animals ·Phase II: Thorough clinical investigation to eliminate investigator bias ·Phase III: Extended clinical evaluation Thalidomide Early 1960’s given to pregnant women to treat morning sickness Later found to cause major birth defects One isomer controls morning sickness, the other leads to birth defects (optical isomer) Administration of drugs Oral ·convenient, effect varies because absorption is affected by stomach content and drug concentration ·absorbed at small intestines ·Rectal ·Effective if a drug cannot be taken orally or if a drug is pH sensitive ·when not able by mouth or destroyed by acids ·Inhalation ·rapid due to extensive network of blood vessels in lungs ·anaesthesia ·Parenteral / Injection ·Subcutaneous (dental, slow) ·Intramuscular (vaccinations, large V) ·Intravenous (fast, practical) · ·Parenteral (injection) ·Subcutaneous ·Beneath the skin ·Slow absorption ·Intra-muscular ·Used when immediate response is not required ·Used for large volumes of drug injection ·Intravenous ·Near instantaneous effect ·Concentration not affected by stomach content ·More about drugs ·Fat-soluble drugs are more easily absorbed, since blood vessels contain a fatty layer ·Capillaries of brain are denser and prevent diffusion of many substances into the brain (bloodbrain barrier) ·Drugs are broken down by the kidneys and liver ·Half-life is the time required for half of the drug to be eliminated ·Toxicity ·LD50 is the dose (in mg of substance per kg of body mass) that is lethal to 50% of laboratory animals ·The lower the LD50, the more toxic the substance ·Lowest LD50 rating known as of yet: botulism toxin (BoTox) – most toxic substance known LD50 of roughly 0.005-0.05 µg/kg ·ED50 – the effective dose where 50% of the population shows a noticeable effect. Examples: aspirin 55 The smaller the ED50, the more effective the substance Examples: aspirin nicotine ethanol (mg/kg) rat: 200 rabbit: 1000 rat: 50 rat: 9000 Therapeutic window = the ratio of LD50 over ED50 The wider the window, the safer the substance Examples: aspirin therapeutic window = 200/55 D.1.4 Terms to Know Therapeutic window: physiological effects that are intended when prescribed. It is also the concentration in the blood where the drug becomes effective but below the level where it would be toxic. · ·Side effects: physiological effects which are not intended. They can vary greatly from drug to drug. ·Tolerance: what occurs when a drug becomes less effective over time. Higher doses are then needed to have the desired effect. Drug effect Main effect (desired) ·Side effects (unwanted responses) · ·Drug effects are relative!! ·Morphine: ·For pain relief, constipation is side effect ·For diarrhoea, pain relief is side effect Tolerance and Dependence Drugs may result in physical or psychological dependence Tolerance means that over time, an individual requires an increased amount of the drug to achieve the same physiological effect ANTACIDS Why is stomach acidic? ·How can we neutralize it? ·What are the most common antacids? ·What are the neutralizing reactions? ·With what can the antacids be combined? · D.2 Antacids Bases (metal oxides, metal hydroxides, metal carbonates, or metal hydrogen carbonates) that react with excess stomach acid to adjust pH · ·Often combined with alginates and antifoaming agents to prevent reflux ·Consumption of too much antacid results in alkalosis (basic stomach) ·Stomach acid (gastric juice) is secreted from gastric glands which line the stomach. The acid (HCl) helps suppress growth of harmful bacteria and aids in digestion by creating a better environment for certain enzymes to work. The following antacids produce a salt, water and carbon dioxide. This can lead to bloating and flatulence. · ·Anti-foaming agents (e.g. dimethicone) are added to help reduce this. CaCO3(s)+ 2HCl(aq)-->H2O(l) +CO2(g) +CaCl2 (aq) NaHCO3(s) +HCl(aq) -->H2O(l) + CO2(g) +NaCl (aq) ·Some antacids also contain alginates. They float on top of the stomach contents, creating a barrier, preventing reflux into the esophagus. Neutralization Reactions Aluminum hydroxide and magnesium hydroxide react with the acid to produce a salt and water. ·Reactions: ·Al(OH)3(s) + 3 HCl(aq) -->AlCl3(aq) + 3H2O(l) · ·Mg(OH)2(s) + 2 HCl(aq) -->MgCl2(aq) + 2H2O(l) ANALGESICS What are soft and strong analgesics? ·How do they prevent pain? ·Aspirin versus paracetamol? ·Morphine versus heroin/codeine? ·Advantages – disadvantages? · Definition of analgesics and categories Analgesics are drugs that relieve pain. These are: Mild analgesics: used for relief of mild pain. (aspirin, acetaminophen) Strong analgesics: used for relief of very severe pain. (morphine, heroin, codeine) Local anesthetics: used as pain killers in localized areas. (lidocaine, procaine) General anesthetics D.3 Analgesics Pain receptors in our bodies are nerves that transmit pain. These are free nerve endings located in various body tissues that respond to thermal, mechanical and chemical stimuli. When stimulated, these pain receptors generate an impulse. The pain results of various impulses arriving at the spinal cord and the brain. ·When tissues become injured, they release chemicals called prostaglandins and leukotrienes that make the pain receptors more sensitive and thus causing pain. · D.3.1 Analgesics Pain relievers act by interfering with pain receptors ·The brain sends nerve impulses through pain receptors and nerve pathways. ·Mild analgesics work by interfering with the pain stimulus at the source. One way is by blocking the production of prostaglandins. These are released when cells are damaged by thermal, mechanical, or chemical energy. · ·Prostaglandins: ·Constrict blood vessels ·Affect hypothalamus (region of brain controls heat regulation and hence fever can result) ·Increase permeability of capillaries to allow for swelling (inflammatory response) Mild analgesics They work by blocking the enzyme-controlled synthesis of prostaglandins. The main effects prostaglandins are: 1) The constriction of blood vessels, which helps increase the body temperature. 2) Direct effect on the body’s heat regulating centre, hypothalamus, which produces fever. 3) Increase of the permeability of capillaries which allows water to pass to the tissue and cause pain and swelling. D.3.2 Mild analgesics ·Aspirin (acetyl salicylic acid or ASA) produced from salicylic acid (relatively strong acid, difficult to take) · ·Addition of acetyl group lowers acidity – less irritating to stomach ·ASA is called a prodrug: a less active form that is converted to the active form after administration ·ASA can also be used to produce alkaseltzer and other drugs by further modification Natural painkillers They are produced naturally in the body. Endorphins and enkephalins are the natural opiates found in the part of the brain and the spinal cord that transmit pain impulses. They are able to bind to neuroreceptors in the brain and produce relief from pain. The temporary loss of pain immediately after an injury is associated with the production of these chemicals. Uses of salicylic acid and its derivatives:· ·Relief from minor aches and pains ·Fever reduction (antipyretic) ·Anti-inflammatory agent ·Anti-clotting agent · As mild analgesic for minor aches and pains. As an antipyretic. As an anti-inflammatory agent when there is swelling from injuries. As an anti-clotting agent in the prevention of abnormal blood clotting and as an anti clotting agent after heart surgery. Disadvantages of aspirin: Can cause upset stomach and ulceration ·Risk of severe gastrointestinal bleeding following alcohol consumption ·Small risk of allergy (.5% of population) ·Accidental infant poisoning; small correlation to Reye’s syndrome in children (a fatal liver and brain disorder with the symptoms of vomiting, lethargy, irritability and confusion.) · Salicylic acid was widely used as a fever reducer However, it is relatively strong acid so it was unpleasant to take orally and it damaged the membranes lining the mouth, esophagus and stomach. · ·Sodium salicylate (its salt) was used but it was also highly irritating to the stomach. ·Its ester called Acetyl Salicylic Acid (ASA) named aspirin retains the beneficial properties of salicylic acid but is less irritating to the stomach. ·ASA is relatively tasteless so it can be taken orally. ·D.3.2 Aspirin substitutes ·Acetaminophen ( paracetemol) ·Does not upset stomach or cause bleeding ·NOT an anti-inflammatory ·Safe in correct dose, but overdose ( >20 tablets) can cause serious liver damage, brain damage, and death) ·Ibuprofen ·Many of the same effects as aspirin but fewer stomach problems ·It is an anti-inflammatory drug. D.3.3 Strong analgesics work by binding to receptors in the brain ·Prevents transmission of pain impulses without depressing the central nervous system · ·The opium alkaloids: ·Opiate: it is a natural or synthetic drug that exerts actions on the body similar to those induced by morphine. ·Narcotic: is a term generally used for drugs that have both a narcotic and analgesic action. morphine, heroin and codeine Morphine is the principal alkaloid and makes up about 10% by mass of raw opium. Codeine makes up about 0.5% by mass of raw opium. Heroin is usually synthesized from morphine and thus is a semi-synthetic drug and it is obtained by relatively simple structural modification of morphine or codeine. Opium plant Morphine ·Strong analgesics ·Opiates (narcotics) ·Opium alkaloids (morphine, heroin, codeine) ·Belong to “opiate” class (drug that exerts actions on the body similar to morphine) or “narcotics” (drug that produces a narcotic (sleep-inducing) effect as well as an analgesic (pain relieving) effect) Morphine is principal alkaloid, making up about 10% by mass of raw opium ·Codeine is about .5% of raw opium ·Heroin (diamorphine)is synthesized from morphine (semi-synthetic drug) via a simple acetylation Note: All three share the following functional groups; benzene ring, ether, alkene, amine (tertiary). ·Codeine and morphine also have a hydroxyl group while heroin has ester functional groups. · Structures D.3.3 Advantages of Opiates: ·Pharmacological effects ·Major effects on: ·Nervous system ·The eye ·GI tract gastrointestinal tract (the digestive system) ·Uses: ·Strong analgesic for relieving severe pain ·Treatment of diarrhea (produces constipation) ·Cough suppressant ·Constriction of the pupil ·Narcotic effects · ·Disadvantages: ·Psychological effects ·Drowsiness, mood change, mental fogginess, nausea and vomiting ·Anxiety, fear, lethargy, sedation, lack of concern, inability to concentrate ·Effects increase from codeine to morphine to heroin (highest) ·Tolerance and Dependence ·Cross-tolerance can occur (users tolerant to one opiate will be tolerant to other opiates) ·Users may not function properly without the drug, experience withdrawal symptoms (addiction) Tolerance appears due to the induction of drug metabolizing enzymes in the liver and also to the adaptation of neurons in the brain to the presence of the drug. The users that became tolerant to one opiate will also exhibit a tolerance to all other opiates. Narcotic Effects ·Short-Term: ·Feelings of well-being and contentment ·Dulling of pain and lessens fear and tension ·Euphoria ·Long-Term: ·Constipation and reduced libido ·Loss of appetite and poor nutrition ·Dependence, Tolerance and Withdrawal Tolerance appears due to the induction of drug metabolizing enzymes in the liver and also to the adaptation of neurons in the brain to the presence of the drug. ·The users that became tolerant to one opiate will also exhibit a tolerance to all other opiates. · The opiates (in general) They are extremely potent and valuable drugs for the treatment of pain They have the capacity of inducing a state of euphoria and relief from physiological pain The opiates induce profound tolerance and physiological dependence They are important both medically and sociologically as the user is difficult to treat and must frequently resort to crime to support the habit and reach a source of supply. Summary of the effects of opiates ·Short term effects ·Sedation and stupor ·Euphoria ·Reduced tension, worry and fear ·Reduced coughing reflex ·Occasional death from overdose ·Long term effects ·Loss of appetite ·Sterility ·Withdrawal illness, loss of job, crime ·Diversion of energy and money ·Risk of dangerous infections due to shared needles DEPRESSANTS What is their effect? ·How is effect dependant on dose? ·Tranquilizers ·Sedatives ·Hypnotics ·Anaesthetics ·Ethanol (effects, detection) ·Most common depressants? · D.4 Depressants (anti-depressants) ·Drugs that calm and relax (depress) the central nervous system ·They alter the activity of neurotransmitters. ·They slow down brain activity ·They slow down heart activity ·They reduce breathing rate ·They dull emotional responses ·The effect depends upon the dosage as well. Low doses have little or no noticeable effects. The progression of dosage effects is as follows: ·Low doses ·Little or no effect ·Moderate doses ·Sedation ·Soothing ·Reduction of anxiety ·High doses ·Sleep ·Extremely high doses ·Comma ·Death Tranquilizers Mild action n Relieve anxiety and tension ·Alcohol, valium, librium (Reduce distress but do not produce sleep) ·Sedatives ·Barbiturates (Reduce distress but do not produce sleep, stronger than tranquilizers) ·Hypnotics ·Chloral hydrate (produces sleep in larger doses) ·Lethal Dose ·Drugs used to treat clinical depression are often called “antidepressants” because they relieve depression. Sedatives Soothing of distress Not producing sleep at normal doses Barbiturates ·D.4.2 Alcohol Small, fat-soluble organic molecule – readily penetrates cell membrane and is easily absorbed from the GI tract ·Social effects: ·Costs ·Sickness and death associated with abuse ·Crime and traffic costs ·Adverse effects to families due to abuse ·Physiological effects ·Short term: ·Reduces anxiety and inhibitions ·Impairs attention, judgment, and control ·Violent or aggressive behavior ·Loss of motor function ·Effect depends on body mass and concentration of alcohol in the blood ·Dehydration, vomiting, loss of consciousness, death (high amounts) · ·Long-term ·Alcoholism is caused by an inability to reduce alcohol intake ·Withdrawal symptoms (nausea, sweating, anxiety, hypertension ·Tolerance ·Cirrhosis (scarring) and cancer of the liver (the major detoxification organ) ·Heart disease ·Hypertension ·Strokes, brain damage ·Gastritis ·Ulcers ·Depression ·Birth defects (fetal alcohol syndrome) D.4.4 Use and abuse of ethanol Synergistic effects ·Alcohol + aspirin - risk of stomach bleeding ·Alcohol + sedatives - heavy sedation, comma, death ·Alcohol + cocaine - high blood pressure, irregular heart beat Doses 30-50mg /100ml of blood Euphoria 100mg / 100ml of blood Slurred speech, staggering, aggressive behavior 200mg / 100ml of blood Difficult movement and vision 400mg / 100ml of blood Comma, death · Alcohol interacts with other drugs (Synergistic effects) ·Can produce coma or death when combined with sleeping pills or barbiturates ·Can cause stomach bleeding with aspirin ·Can inhibit breakdown of other drugs by the liver ·May increase the risk of cancer when used with tobacco ·Measuring blood alcohol concentration (BAC) 3 ·Mass (g) of ethanol per 100 cm of blood 3 ·.08% is legal limit in US (.080 g per 100 cm of blood) ·Ethanol is easily absorbed from the stomach to the blood, where it is exhaled by the lungs (ethanol is fairly volatile) · C2H5OH(l) C2H5OH(g) ·The alcohol vapor can be detected by a number of methods · D.4.3 Breathalyzer test ·Subject breathes into an analyzer containing an oxidizing agent (potassium dichromate (K2Cr2O7)) and a detector ·Oxidizes ethanol to ethanoic acid ·This is an oxidation-reduction reaction that involves an electron transfer ·This electron transfer generates an electric current which can be detected by the machine or produces a color change (orange to green) detected by a photocell ·Unreliable in legal cases · Detection of ethanol Chromatography (GLC) Ethanol in breath, blood and urine Inert gas through liquid or solid Compounds separated by b.p. Different retention times recorded Amount = area under peak Other drugs can be detected, too · Gas Liquid Chromatography ·More precise than breathalyzer ·Uses a stationary phase (non-volatile liquid or solid support) and a mobile phase (inert gas, like N 2) ·Breath components (CO2, H2O, and alcohol vapor) are injected into the machine and partitioned (divided) between the stationary and mobile phases ·Or blood or urine components are vaporized, mixed with inert gases and injected into the machine over the surface of a non-volatile liquid ·Components exit at different intervals (each substance has a different affinity and bond strength for the two phases, and thus move through at different rates) ·Components are detected ·Retention time for each component is measured (time taken for each component to pass through the column) ·Blood alcohol’s retention time is compared to the retention time for a standard ethanol sample Detection of ethanol Intoximeter (infra-red spectroscopy) Ethanol in breath Transmittance versus wavenumber =IR spectrum -1 Characteristic peak of -OH @ 3340cm Comparison of sample and reference Amount = size of peak ·Infra-Red Spectroscopy ·IR light does not promote electrons to higher levels, but does provide enough energy to make molecules “vibrate” ·Vibrational motion depends on the mass of the molecule and the types of bonds present ·IR spectrum therefore depends on types of molecules present (“molecular fingerprint”) ·Scale is based on wavenumber (1/wavelength) ·Police use intoximeter (IR spectrometer) to confirm breathalyzer test ·IR radiation is passed through breath sample ·C-H group in alcohol absorbs a certain frequency of IR light ·% transmittance of the C-H frequency is determined, indicating amount of alcohol present ·A different version of the intoximeter uses a fuel cell which oxidizes the ethanol, and a detectable amount of electrical voltage is detected. (Quite accurate) ·D.4.5 Other Depressants Diazepam (Valium) is a tranquilizer used to relieve anxiety and tension ·Nitrazepam (Mogadon) – sleeping pills, is a hypnotic drug used to induce sleep ·Fluoxetine hydrochloride (Prozac) – antidepressant, is used to treat mental depression by increasing activity of serotonin (a neurotransmitter) ·Be familiar with the general structures of these three molecules. See Data Booklet, Section 20. · Structures STIMULANTS What is their effect? ·Adrenaline versus amphetamines ·Nicotine ·Short-term effects ·Long-term effects ·Caffeine ·Effects ·Comparison to nicotine · D.5 Stimulants Stimulate brain and central nervous system ·Alters the levels of neurotransmitters ·Effects: ·helps facilitate breathing by relaxing air passages ·Cause increased alertness and awareness ·Reduces appetite ·Can cause palpitations and/or tremors ·Can cause restlessness, sleeplessness, fits, delusions, and hallucinations · ·Include amphetamines, nicotine, and caffeine D.5.2 Adrenaline (epinephrine) is a natural stimulant produced in the adrenal gland. ·it is released when in stress or fear ·it controls ·- heart/breathing rate ·- pupil dilation ·- sweating · Amphetamines Have structures similar to adrenaline (epinephrine) Both are derived from Phenylethylamine Mimic the actions of adrenaline (sympathomimetic) Constrict arteries, increase sweat production, increase heart rate, blood pressure, respiration Amphetamine stimulant ·Suppresses appetite ·Treats narcolepsy and attention deficit hyperactivity disorder (ADHD) · • Short-term effects: - increase in heart rate and breathing - insomnia - dilation of the pupils - decrease in appetite - possible fatigue and depression • Long-term effects: - weight loss - constipation - emotional instability - dependence Ecstasy potent stimulant designer drug can be fatal neurotoxic Phenylethylamine (love molecule) “high” feeling of lovers also found in chocolate Dopamine Transmits to neurons signals of joy, happiness, excitement Methamphetamine (“speed”) potent stimulant very addictive severe withdrawal symptoms “meth mouth” D.5.3 Nicotine ·Initial stimulant effect, followed by depression, which encourages frequent use ·Short term effects: ·Increased heart rate and blood pressure, putting stress on the heart ·Reduces urine output ·Long term effects ·Increased risk of heart disease and blood clot (thrombosis) ·Inhibits oxygen-carrying capacity of blood ·Increased risk of peptic ulcers · NICOTINE One third of the world’s population is addicted to smoking because tobacco contains nicotine ·Nicotine produces psychological and/or physical dependence ·Nicotine builds up tolerance ·Stopping smoking can produce temporary symptoms like a craving for tobacco, nausea, weight gain, insomnia, irritability and depression · Short-term effects: stimulates nervous system ·increases heart rate and blood pressure ·increases concentration ·constricts blood vessels ·stresses heart ·reduces urine output · ·Long-term effects: ·increases risk of heart disease coronary thrombosis ·inhibits ability of blood to carry oxygen (CO) ·excess acidity > peptic ulcers ·mouth/lung cancer ·adverse effects on pregnancy Smoking can also lead to ·Lung cancer ·Cancer of the larynx and mouth ·Heart and blood vessel disease ·Empyhsema ·Chronic bronchitis ·Air pollution ·Fires!! ·Stained fingers and teeth ·Bad breath ·Very easy to develop dependence on nicotine compared to alcohol or barbiturates ·Withdrawal symptoms: weight gain, nausea, insomnia, irritability, fatigue, depression, and inability to concentrate · D.5.4 Caffeine ·Increases rate of cellular metabolism and therefore respiration ·In low doses, enhances wellbeing, alertness, energy, and motivation ·In large amounts, physical coordination and timing are affected, and sleeplessness may also result. ·Weak diuretic (increases urine flow) ·Tolerance occurs, but no physical dependence ·Vasoconstrictor (blood vessel constriction), so can help in treating migraines ·Can help newborn babies to breathe as it increases respiration · CAFFEINE It is the most widely used stimulant in the world. ·It is present in coffee, tea, chocolate and cola drinks and is also found in some pain killers or other medicines. ·People that consuming 400 mg of caffeine a day may have dependence & physical side effects. · Caffeine, like nicotine, contains a tertiary amine group (nitrogen atom attached to three organic [i.e. carbon-containing] substituents): · • Like nicotine, morphine, codeine and cocaine, caffeine is also an alkaloid. • Alkaloids are nitrogen-containing compounds of plant origin containing heterocyclic rings and a tertiary amine group. • Theobromine has a similar structure to caffeine, which is also found in chocolate. (It does not contain bromine!) theobromine Effects at low doses: - respiratory stimulant - weak diuretic - enhances concentration and alertness - reduces migraines (constriction of blood vessels) • Effects at high doses: - anxiety - irritability - sleeplessness ANTIBACTERIALS ·The discovery of penicillin ·How penicillin works ·Modifications of penicillin ·Use and overprescription ·Broad versus narrow spectrum antibiotics Drugs that prevent the growth of, or kill, microorganisms that cause infectious diseases. Microorganisms = single celled life forms capable of independent life if given a required amount of nutrients. infectious diseases = Occur when the body’s natural defenses are ineffective due to 1) lack of natural immune system against infection. 2) too many microorganisms for the body’s immune system to overcome. 3) rapid growth of the microorganisms. D.6 Antibacterials Antibacterials are selective: they attack infectious bacteria rather than human cells ·These drugs are selective, they are ineffective against normal body cells. ·Can be ·Bacteriostatic (inhibit bacterial cell division) or ·Bacteriocidal (directly kill bacteria) ·Normally ineffective against viruses because viruses live within host cell, which are unaffected by most antibiotics ·The most well known antibacterials are penicillins. The discovery was by accident, sometimes called a serendipitous event. · Infectious Agents There are two types of infectious agents: ·Bacteria ·Viruses ·Antibiotics are ineffective against viruses since they incapable of combating normal body cells. ·Antibiotics aid white blood cells by ·1. Preventing bacteria from multiplying ·2. Preventing cell division (bacteriostatic drugs) ·3. Directly killing Bacteria (bacteriocidal drugs) ·Examples of bacterial infections: tetanus, tuberculosis (TB), cholera, etc. ·Examples of Viral infections: influenza, common cold, hepatitis, etc. · D.6.1 Penicillins: ·Produced from fungi (penicillium genus) ·Accidentally discovered by Alexander Fleming, who noticed that bacteria did not grow around a spot of penicillium notatum mold on a culture plate ·Fleming could not isolate the “penicillin,” and later gave up the research ·Florey and Chain, at Oxford, renewed the research and started administering the drug to humans ·Awarded the Nobel Prize ·Thousands of lives were saved during WWII · History of Penicillins 1890s: Found out that certain fungi killed bacteria. 1928: Alexander Fleming finds out that the mold penicillium notatum prevented the growth of the bacteria staphylococcus aureus. 1940: Florey and Chain used penicillin on mice. 1941: Penicillin used for the first time on a human being. 1943: Penicillin available clinically. 1945: Fleming, Florey and Chain receive Nobel prize. D.6.2 Structure Penicillins all have a certain structural feature in common, the 6-APA group (6-aminopenicillic acid) Structure has no effect on bacterial growth, but when an extra side chain is added to the amino (NH2) group, it becomes “active” Side chain varies between different types of penicillin: Penicillin G, the first type created, is not acidresistant, and must be injected to bypass the stomach Penicillin V is acid-resistant Cloxacillin is acid and penicillinase (bacteriaproduced enzyme that breaks down penicillin) resistant Structure of Penicillin Penicillin G (first penicillin used): ·deactivated by ·1) stomach acid injected into body. ·2) Penicillinase, an enzyme created by bacteria ·Penicillin V: acid resistant penicillin created by modifying side chains. ·Active penicillin: Aminopenicillanic acid, 6 APA, (common in all penicillins) and a sidechain: · C6H5-CH2-: benzyl penicillin or penicillin G · C6H5-CH2-CH2-:penicillin V · Cloxacillin, effective against pencillinase and acid. · Penicillins differ only in their type of side chain ·Altering the side-chain makes them more resistant to the penicillinase enzyme. · Penicillins function by interfering with the cross-links (chemicals) that connect separate layers of the bacterial cell wall ·Cell wall is weakened and the bacterial cell bursts, killing the bacteria · ·Humans' cells do not have cell walls and are thus unaffected by penicillins Bacteriocidal Drugs Bacteria have cell walls mainly composed by polysaccharides that protects their cell structure and inside components. These cell walls are strong due to the chemical cross-links. ·How does it work?: ·1. Penicillins interfere with cell wall construction of bacteria. ·2. The cross links are destroyed, hence weakening the cell walls. ·3. Bacteria is unable to hold its size and shape. ·4. Water enters by osmosis, the cell expands and bursts ·5. Bacteria is killed by this. · Disadvantages of penicillins ·1. Small percentage of the population (10%) experience allergic reactions and other side effects such as body rash. ·2. If used repeatedly, it may wipe out harmless or helpful bacteria. In addition these bacteria that are wiped out may be replaced by harmful bacteria. ·3. Genetic resistance of bacteria. If antibiotics are used extensively some bacteria survive and pass on their immunity to next generations. Such examples are Typhoid, Gonorrhoea and Malaria. ·A microorganism may become resistant as a result of mutation. A mutated bacteria may produce an enzyme that makes antibiotics ineffective. ·Result of these mutations: Need for constant renewal of antibiotics. Hence, antibiotics should only be used when no other treatment is effective. · D.6.3 Side effects Antibiotics Side effects include fever, body rash, shock, and death ·Over prescription can result in destruction of harmless bacteria in the digestive tract, allowing harmful bacteria to colonize ·Over prescription leads to genetics resistance over time, rendering the antibiotic eventually useless ·Superbugs, such as antibiotic resistant TB (tuberculosis), are becoming increasingly common. A combination of several drugs are sometimes required in these cases. · ·Thus, antibiotics should only be prescribed when there is no other option that can reduce suffering or save a life Broad vs. Narrow Spectrum Antibiotics: ·Broad spectrum ·Effective against a wide variety of bacteria ·Tetracyclines (Aureomycin, Terramycin),‘Mycin’ is the suffix used for antibiotics obtained from soil fungi. ·Ampicillin ·Repeated use may wipe out harmless or helpful bacteria in the digestive tract, which may be replaced by harmful strains ·Narrow spectrum ·Effective against only certain types of bacteria ·Penicillins (and sulfa drugs) · ·Typically, a broad spectrum is initially prescribed until the bacteria can be identified, at which point a narrow spectrum is prescribed ·Humans also have an important role. Patient compliance is also required to help with the resistance issue. Patients should take the full course of the antibiotic as prescribed to them. ·Antibiotics in animal feed ·Antibiotics are added to animal feed to prevent the spread of infection throughout livestock ·However, this can encourage the development of drug-resistant bacteria that humans will eventually be exposed to ANTIVIRALS Viruses versus bacteria ·How do antiviral drugs work? ·What is HIV and what AIDS? ·Why is it difficult to fight HIV? ·(AIDS prevention methods?) · D.7 Antivirals Viruses are submicroscopic, non-cellular infectious particles that can only reproduce inside a living host cell ·Unlike bacteria, which have a cellular structure, viruses have no nucleus, cytoplasm, or cell membrane ·This limits the effectiveness of antibacterial drugs on viruses · ·D.7.2 Controlling viruses ·Antibacterials may be effective if they block the transfer of genetic information, although few do ·Vaccination is primary method of prevention ·Patient is exposed to weakened or inert viral particles to stimulate immune system ·Immune system produces antibodies, crucial in the immune response, specific to that virus ·Future exposure to active viral particles is more easily controlled because antibodies have already been produced against it ·Ex: measles, meningitis, polio, AIDS, avian flu Many antiviral drugs work to inhibit the function of replication-specific enzymes. ·Some alter the cell’s DNA so that the virus can not use it to multiply. ·Progression of the disease is halted but the virus is not completely eradicated from the body. Ex: cold sores from herpes infections · ·Latent viruses are viruses that inject their genetic material into a host cell, but the material is not expressed until a later date ·Herpes simplex virus, certain types of cancer D.7.3 AIDS virus The HIV virus is the that leads to the condition we call AIDS. · ·Attacks immune system by binding to a receptor glycoprotein (CD4) on T4 immune (white blood) cells ·Difficult to fight because of: ·its ability to mutate (thus rendering a previous treatment ineffective) ·Its metabolism is similar to human cells ·Virus mutates rapidly ·It may lay dormant for a period of time, making it difficult for the immune system to fight. ·As of now, effective vaccines, antiviral, and anti-retroviral drugs have not yet been developed. ·Access to these very expensive treatments become increasingly difficult for the hardest hit areas of the World mainly due to socioeconomic conditions AIDS/HIV ·What is HIV? ·Human Immunodeficiency Virus. ·HIV attacks the immune system - the part of our body that protects us from infections and illnesses. It takes about 3 - 6 months for HIV to appear after infection What is AIDS? · AIDS, the Acquired Immune Deficiency, is a disease caused by HIV. Once infected with HIV, it typically takes 10 years to develop AIDS (although in some, this time may be shorter). People with AIDS cannot fight common diseases, and therefore become very ill and die. A person can be infected with HIV and not have AIDS HIV/AIDS Infection: How do you get it? ·Unprotected sexual intercourse - anal, vaginal, oral ·Sharing drug needles and syringes ·Sharing unsanitary piercing instruments ·From mother to child ·Blood contact HIV is NOT spread via… Kissing ·Biting ·Blood sucking insects · Can I tell if my partner is sick? People who are infected can have the virus in their body for years before getting sick. During this time, they look and feel healthy. If they have sex with anyone, there is the chance that they will spread the virus to their sex partner. · The College Facts ·One in every 500 American college students is infected with HIV ·The rate of HIV infection in the general American population is one in 250 ·4 factors that put college students at increasing risk: peer pressure, lack of maturity, increase in alcohol/drug use, and growing incidence of date rape