Biology
advertisement

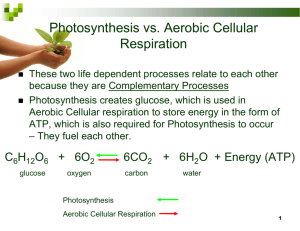
Academic Biology: Midterm Review Quarter #1 Chapter 1: The Science of Biology Biology – the study of the living world Scientific Method Step 1: Observation Step 2: Forming a Question Step 3: Form a Hypothesis (suggested answer based on evidence) Step 4: Experiment & Compile Data (should only test ONE variable Step 5: Analyze Data Step 6: Draw Conclusion & Retest as needed Chapter 1: The Science of Biology Independent Variable The variable the scientist changes Dependent Variable The variable being measured Use the metric system When enough experimental data support a hypothesis, it can become a theory. Theories change! Chapter 1: The Science of Biology 8 Characteristics of Living Things: 1. Made up of cells (smallest unit of life) 2. Reproduce (sexually or asexually) 3. Based on universal genetic code (DNA) 4. Grow & develop 5. Obtain & use materials/energy (metabolism) 6. Respond to their environment (stimuli) 7. Maintain stable internal environment (homeostasis) 8. Change over time (evolution) Chapter 1: The Science of Biology Tools for Observing Life: Compound Light Microscope – to observe small, living samples Electron Microscope – higher magnification, but can only observe dead, properly preserved/prepared samples Robert Hooke coined the term “cell” looking at cork through a simple light microscope Chapter 2: The Chemistry of Life 4 Main Organic (Carbon) Compounds: Carbohydrates Main source of energy Made up of monosaccharides (sugars) Proteins Control reaction rates, allow molecules in and out of cells, fight disease, make up muscles Made up of amino acids Chapter 2: The Chemistry of Life 4 Main Organic (Carbon) Compounds: Lipids Comprise membranes, energy storage, insulation Made up of glycerol & fatty acids Nucleic Acids Store and transmit genetic information Made up of nucleotides (sugar, phosphate group & nitrogenous base) Chapter 2: The Chemistry of Life Chemical Reactions – rearranging of atoms to form new compounds Activation Energy – E needed to start a rxn Catalyst – speeds up the rate of chemical reactions Enzymes – naturally occurring, biological catalysts; lower activation E needed Can only be used to speed up ONE specific reaction!!! Chapter 2: The Chemistry of Life Water Polarity H’s and O don’t share e-’s evenly O side ends up slightly negative; H side is slightly positive Universal solvent Ice floats! Most abundant compound in living things Hydrophilic – water loving Hydrophobic – water fearing Chapter 2: The Chemistry of Life Acids Donates H+’s pH <7 Bases Absorb H+’s pH >7 Quarter #2 Early Earth The Universe Formed by Big Bang 14 BYA The Earth Formed 4.6 BYA First organisms were probably bacteria (archaebacteria) Reducing atmosphere oxidizing atmosphere Cyanobacteria released O2 Early Earth Miller & Urey Recreated hypothesized conditions of early Earth in lab Demonstrated how organic molecules could have formed before life was present Chapter 16: Darwin’s Theory of Evolution Evolution by Natural Selection – species change over time Struggle for Existence Survival of the Fittest Natural Selection Evidence of Common Ancestry: Structural similarities (body parts), similar genes, similar species in geographically isolated areas Chapter 16: Darwin’s Theory of Evolution Published The Origin of Species Controversial for its time Fossil Record – collection of fossils over time that illustrates ancestry commonalities between species Shows that most organisms that have ever lived on Earth are extinct! Chapter 7: Cell Structure & Function In multicellular organisms, cells specialize to perform different functions. Cell Theory: 1. All living things are made of cells. 2. Cells are the basic unit of life. 3. All cells are produced by existing cells. Chapter 7: Cell Structure & Function Prokaryotic Cells – lack “true” nucleus and membrane-bound organelles (bacteria) Eukaryotic Cells – contain a membrane-bound nucleus as well as specialized organelles Plant Cell – square-ish; cell wall, large central vacuole, chloroplasts & general organelles Animal Cell – round-ish; centrioles & general organelles (Do you remember the functions of each organelle?) Chapter 7: Cell Structure & Function Endosymbiotic theory Proposed by Lynn Margulis Bigger prokaryotes engulphed smaller prokaryotes organelles 3 endosymbionts: chloroplast, mitchondria, and centrioles Fluid Mosaic Model of the Cell Membrane: Phospholipids – main component Proteins channels and pumps – made from proteins; aid materials in movement across cell membrane Cell Transport: Passive transport Diffusion – movement from an area of high concentration to an area of low concentration Osmosis – movement of water from an area of high concentration to an area of low concentration Facilitated Diffusion – Diffusion that gets “help” from a protein Active Transport Movement from LOW to HIGH concentration Requires energy Endocytosis: Pino- and phagocytosis Exocytosis Water movement Hypertonic – water out; cell shrinks Hypotonic – water in; cell may burst Isotonic – water in and out; cell’s happy! Energy Transfers & ATP ATP ADP + Pi + E Energy is released when the bond holding the end phosphate is broken The ATP Cycle ATP Energy for cells E from food ADP + Pi Energy Pyramid E 4° con. 3° consumer 2° consumer 1° consumer 1° producer % E/g decreases as you go up because efficiency in E transfer is not 100% E is lost - Metabolism, waste, cellular processes Chapter 8: Photosynthesis 6CO2 + 6H2O + Sunlight C6H12O6 + 6O2 Occurs in the chloroplast Thylakoid: membrane sacs; Chlorophyll (pigment) found in membranes (reflects green light) Light-Dependent Reactions – produce high-energy molecules (ATP & NADPH) Stroma: fluid surrounding thylakoid Calvin Cycle (Light-Independent Reactions) – produce high-energy sugars Chapter 9: Cellular Respiration 6O2 + C6H12O6 6CO2 + 6H2O + 36 ATP 2 Types: Aerobic Anaerobic Chapter 9: Cellular Respiration Steps of Aerobic Respiration Glycolysis – occurs in cytoplasm, net gain 2 ATP, anaerobic (no O2 needed), if O2 is present followed by Krebs Cycle Krebs Cycle - occurs in matrix (inner cavity of mitochondria), aerobic (O2 needed), net gain 0 ATP Electron Transport Chain – occurs in cristae (mitochondria), aerobic (O2 needed as the final electron acceptor), net gain 34 ATP Chapter 9: Cellular Respiration Fermentation Alcoholic Fermentation Lactic Acid Fermentation – causes muscle soreness during excessive physical activity Sources of Energy: Stored ATP (energy is released as bonds are broken and phosphate groups are removed from ATP) Cellular Respiration Lactic Acid Fermentation Questions???