Lecture4
advertisement

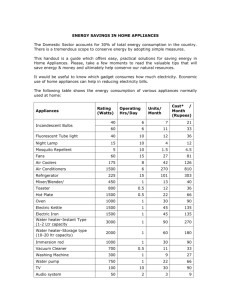
Power use per person per day, by Country (2007) Why do we use the unit of the “Joule”? Why do we use the unit of the “Joule”? The physicist James Joule (1843) discovered that the amount of energy needed to raise the temperature of water was the same whether it was work done on the water or heat added. Joule’s mechanical heating apparatus (1845) First Law of Thermodynamics: Heat = Work W = ΔE = ΔTE So, how much heat do you need to add to raise the temperature of 1 gram of something 1°C? It depends on the substance!!! Q = mcΔT Heat added = (mass)(specific heat)(change in T) What are some consequences of this for climate and climate change? THIS is how the “calorie” and “Btu” were defined! 1 calorie = energy required to raise 1 gram of water 1°C 1 Btu (British thermal unit) = energy required to raise 1 pound of water 1°F THIS is how the “calorie” and “Btu” were defined! 1 calorie = energy required to raise 1 gram of water 1°C 1 Btu (British thermal unit) = energy required to raise 1 pound of water 1°F It takes 4.19 kJ to raise 1 kg of water 1°C It takes a LOT of energy to get water to change its phase (to get ice to melt, and to get liquid water to boil)! It takes a LOT of energy to get water to change its phase (to get ice to melt, and to get liquid water to boil)! Heat of Fusion: It takes 335 kJ to turn 0°C ice into 0°C water Heat of Vaporization: It takes 2260 kJ to turn 100°C water into 0°C steam How much energy does it take to turn 1 kg of 0°C ice into 100°C steam? How much energy does it take to turn 1 kg of 0°C ice into 100°C steam? E = 335kJ + (4.19 kJ/°C)(100°C) + 2260 kJ = 335 kJ + 419 kJ + 2260 kJ = 3014 kJ HEAT flow by three mechanisms: Conduction Convection Radiation Fig. 4-7, p. 106 CONDUCTION: k = thermal conductivity CONDUCTION: R = δ/k = “R value” Qc = A x (T2 – T1) t R Table 5-2a, p. 134 Table 5-2b, p. 134 Equivalent thicknesses needed to provide an R value of R = 22 (ft2hr°F/Btu) CONVECTION: RADIATION (Electromagnetic): RADIATION (Electromagnetic): RADIATION (Electromagnetic): Many energy-related machines are examples of a “Heat Engine” Transforms Heat into Work Ex/ Steam engines, combustion engine, heat pump (refrigerator or air conditioner) Efficiency = Work out x 100% Energy in Efficiency = Work out x 100% Energy in = (Heat in – Heat out) x 100% Heat in Efficiency = Work out x 100% Energy in = (Heat in – Heat out) x 100% Heat in = (TH – TC) x 100% TH Efficiency = (TH – TC) x 100% TH The HOTTER an engine runs, the more efficient it is. Efficiency = (TH – TC) x 100% TH The HOTTER an engine runs, the more efficient it is. Ex/ If TH = 400K and TC = 300K then Max possible efficiency = (400K – 300K)/400K = 25% Efficiency = (TH – TC) x 100% TH The HOTTER an engine runs, the more efficient it is. Ex/ If TH = 400K and TC = 300K then Max possible efficiency = (400K – 300K)/400K = 25% Ex/ If TH = 1000K and TC = 300K then Max possible efficiency = (1000K – 300K)/1000K = 70% Efficiency = (TH – TC) x 100% TH The HOTTER an engine runs, the more efficient it is. Ex/ If TH = 400K and TC = 300K then Max possible efficiency = (400K – 300K)/400K = 25% Ex/ If TH = 1000K and TC = 300K then Max possible efficiency = (1000K – 300K)/1000K = 70% It can never be 100%! Second Law of Thermodynamics 1) Heat can flow spontaneously (by itself) only from a hot source to a cold sink. Second Law of Thermodynamics: 1) Heat can flow spontaneously (by itself) only from a hot source to a cold sink. 2) No heat engine can be 100% efficient; some heat always has to be discharged to a sink at a lower temperature Which of these will happen? Second Law of Thermodynamics: In ANY system, the result of an interaction will be an INCREASE in disorder (Entropy) Time has a direction! (toward disorder) Which of these will happen? How does an air conditioner work? How does an air conditioner work? What happens to a gas when you compress it? A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. 1. HOT A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. 2. Cools off 1. HOT A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. 2. Cools off 1. HOT 3. Ambient A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. 2. Cools off 1. HOT 3. Ambient 4. COLD A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. 2. Cools off 1. HOT 5. Warms up 3. Ambient 4. COLD A heat pump (air conditioner, refrigerator) uses the trick that gases become hot when compressed, cold when expanded. 6. Ambient 2. Cools off 1. HOT 5. Warms up 3. Ambient 4. COLD You can also do this by changing the phase – from liquid to gas and back again. You can also do this by changing the phase – from liquid to gas and back again. The gas GIVES OFF heat when you compress it into a liquid You can also do this by changing the phase – from liquid to gas and back again. The gas GIVES OFF heat when you compress it into a liquid The gas ABSORBS heat when you let it expand back into a gas What are three things about the world’s energy future that most concern you? What are three things about the world’s energy future that make you optimistic? 1) 1) 2) 2) 3) 3)