Thermochemistry ppt 1/28

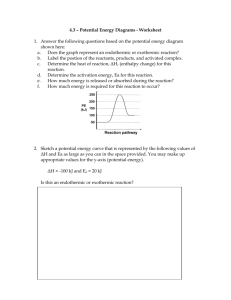
Thermochemistry
Definitions
• Energy – capacity for doing work or supplying heat.
• Thermochemistry – study of energy changes that occur during phase changes and chem. rxns.
• Chem. Potential Energy – energy stored in chemical bonds.
Lots of energy stored in bonds!
Example
Energy difference.
5473 kJ/mol
Little energy stored in bonds.
Heat
• Represented by q .
• Energy that transfers from one object to another because of a Temp. difference between them.
• Heat flows from warm cool until the two objects are at the same Temp.
Exothermic vs. Endothermic
• In exothermic processes, the system loses heat as its surroundings warm up.
– q has a negative value b/c the system is losing heat.
• In endothermic processes, the system gains heat as its surroundings cool down.
– q has a positive value b/c the system is gaining heat.
Potential Energy Diagram of Ice
Melting at 0ºC.
Water
Is the melting of ice an endothermic or an exothermic process? How can you tell?
Ice
Time
Measuring Heat Flow
• SI Unit of heat flow:
Joule (J)
• Common unit used in chemistry: calorie
(cal)
– Amt. of heat needed to raise 1 gram of water by 1ºC.
– 1 cal = 4.184 J
• Food Calorie (capital “C”) = 1000 cal, or 1 kilocalorie = 4184 J
Heat Capacity
• Amount of heat needed to raise an object’s temperature by 1°C.
– Depends on the chemical composition and the mass of the object.
• EXAMPLE: 1 gram of water requires 1 cal to raise its temperature by 1°C.
– 100. g of water require 100. cal to raise the temp. by 1°C.
Heat Capacity
Same temperature change
10 g H
2
O
1 g H
2
O
Specific Heat (c)
• Amt. of heat needed to raise 1 gram of a substance’s temperature by 1ºC.
– Expressed in J/g ºC, or cal/g ºC
• The higher a substance’s specific heat, the more energy it takes to heat it.
• Substance’s with low specific heats heat up and cool down quickly (most metals, e.g.)
Some Specific Heats
Substance
Water
Grain alcohol
Ice
Steam
Chloroform
Aluminum
Iron
Silver
Mercury
1.7
0.96
0.90
0.46
0.24
0.14
4.18
2.4
2.1
Specific Heat
J/gºC cal/g ºC
1.00
0.58
0.50
0.40
0.23
0.21
0.11
0.057
0.033
Specific Heat (c)
• c = heat / (mass x change in Temp.)
• c = q / (m x ΔT)
• q = m x c x ΔT
Example Problem
• The temperature of a 95.4-g piece of Cu increases from 25.0ºC to 48.0ºC when the
Cu absorbs 849 J of heat. What is the specific heat of Cu?
– SOLUTION: q = m x c x ΔT
• 849 J = (95.4 g) c (48.0ºC – 25.0ºC)
• 849 J = (95.4 g) c (23.0ºC)
• 849 J = (2190 gºC) c
• c = 0.388 J/gºC
• Based on what you know about metals, does this answer make sense?
Example Problem
• When 435 J of heat is added to 3.4 g of olive oil at 21ºC, the temperature increases to 85ºC. What is the specific heat of olive oil?
– SOLUTION: q = m x c x ΔT
• 435 J = (3.4 g) c (85ºC – 21ºC)
• 435 J = (3.4 g) c (64ºC)
• 435 J = (220 gºC) c
• c = 2.0 J/gºC
Example Problem
• How much heat is required to raise the temperature of 250.0 g of mercury by
52ºC? The specific heat of mercury is 0.14
J/gºC.
– SOLUTION: q
= m x c x ΔT
– q = (250.0 g)(0.14 J/gºC)(52ºC)
– q = 1800 J = 1.8 kJ
Enthalpy Changes
• Enthalpy (H) – the heat content of a system at constant pressure.
• Enthalpy change (ΔH) – the heat that enters or leaves a system at constant pressure.
• q = ΔH
• Neg. ΔH = exothermic process
• Pos. ΔH = endothermic process
Thermochemical Equations
• Enthalpy change can be written as a reactant or a product.
– Reactant endothermic
– Product exothermic
• Example: The reaction of calcium oxide with water is exothermic.
– It produces 65.2 kJ of heat per mole of CaO reacted.
– CaO(s) + H
2
O(l)
Ca(OH)
2
(s) + 62.5 kJ