Provedení, principy činnosti a základy výpočtu pro výměníky tepla
advertisement

CH3
CHEMISTRY E182019
Nomenclature
of compounds
(names and
formula)
Mixtures.
Balances.
This course is approximately at this level
Rudolf Žitný, Ústav procesní a
zpracovatelské techniky ČVUT FS 2010
CH3
MOLECULES
Molecule is a configuration of atoms in precisely defined ratio bonded together by
ionic or covalent forces. Molecular mass is the sum of atomic masses multiplied by
corresponding number of atoms.
Example: MC6H6=6x12+6x1=78 g/mol
CH3
BINARY COMPOUNDS
Binary compound consists of n-atoms A and m-atoms B, formula AnBm.
Binary compound is electrically neutral from outside, therefore n,m must be related by
oxidation numbers of elements OA and OB
n . OA + m . O B = 0
Oxidation numbers are not unique for different elements (some elements have
different oxidation numbers in different compounds), however there are some rules,
related to corresponding columns in periodic table.
Elements in 1.column have always OA= +1 (alkali metals are donators of electron and become cations)
Elements in 2.column have always OA= +2
Elements in 13.column have usually OA= +3 (example Al aluminium)
Elements in 16.column have usually OB= -2 (nonmetals, example O,S)
Elements in 17.column have OB= -1 (halogens F,Cl,Br,I are acceptors of electron and become anions)
Example: Fe2O3 Iron is in the column 8 (variable oxidation number, not known in advance), however O
has oxidation number -2, therefore the oxidation number of iron must be +3 in this compound.
CH3
BINARY COMP. COVALENT
Nomenclature of binary compounds AmBn (both A,B are nonmetals)
Binary NONMETALS with covalent bonds ALWAYS use PREFIXES (di-, tri-…)
Write more metallic element (with positive oxidation number) first.
Examples
SiC Silicon carbide (or monosilicon monocarbide)
Evaluate oxidation number from electronegativities (look at the table) eSi=1.7, eC=2.5 therefore carbon
is “stronger”, attracts 4 missing electrons and becomes negative with oxidation number OC=-4, while
OSi=4 and balance is preserved -4+4=0 . Element with positive oxidation number is the first (CSi-wrong)
CS2 Carbon disulfide (monocarbon disulfide)
Electronegativities eS=2.4,
N2O5
NO
ClO2
NO3
eC=2.5
Sulphur in column 16, therefore OS=-2, OC=4
Dinitrogen pentoxide
Nitrogen monoxide
Chlorine dioxide
Nitrogen trioxide
SF6
4-2x2=0
Sulfur hexafluoride
SO2 Sulfur dioxide
OF2 Oxygen difluoride
CH3
BINARY COMPOUND IONIC
Nomenclature of binary compounds AmBn (A-metal, B-nonmetal)
Binary METAL+NONMETAL (ionic bonds) WITHOUT PREFIX, use ROMAN
numerals for variable oxidation number of metal Fe2++, Fe3+++, Cu2++, Cu+.
Write always the metal first.
Examples
NaCl sodium chloride
MgO magnesium oxide
MgBr2 magnesium bromide (and not dibromide, oxidation number of Mg is always 2)
Al2O3 aluminium oxide (without prefix, because oxidation numbers are unique)
CuO copper(II) oxide (this is the case, when oxidation number of metal is variable and must
be determined from oxidation number of oxygen – 2, expressed as roman II)
Cu2O
Copper(I) oxide
Fe2O3
CaCl2
Iron(III) oxide
Calcium chloride
CH3
Polyatomic IONs
Anions (negative electrical charge)
F fluoride
Cl chloride
Br bromide
I iodide
CO3 - carbonate (two negative charges)
HCO3 bicarbonate
PO4 - - - phosphate (three negative charges)
O2 - peroxide
OH hydroxide
CH3COO - acetate
NO2- nitrite, NO3- nitrate (ion with less oxygens ends with –ite, otherwise –ate)
SO3 - - sulfite, SO4 - - sulfate
ClO hypo chlorite, ClO2 chlorite, ClO3 chlorate, ClO4 perchlorate
Cations (positive electrical charge)
NH4+
H3O+
amonium
hydronium
CH3
Compounds with Polyatomic IONs
Common (unsystematic) names used by chemists (derived from names of ions)
Examples
Na2SO4
Na2SO3
NH4Br
NaOH
sodium sulfate (more oxygens use –ate, systematic name sodium tetraoxosulfate)
sodium sulfite (less oxygens use –ite)
amonium bromide
sodium hydroxide
Ca(OH)2
(NH4)2SO4
H2CO3
Cu2CO3
NH4NO3
KCN
Fe3(PO4)2
Al(NO3)3
NH4Cl
CaCO3
HNO3
Calcium hydroxide
Ammonium sulfate (and not sulfite)
Hydrogen carbonate
Copper(I) carbonate
Ammonium nitrate
Potassium cyanide
Iron(II) phosphate
Aluminium nitrate
Ammonium chloride
Calcium carbonate (limestone)
Hydrogen nitrate (nitric acid)
CH3
Inorganic ACIDS and salts
Common (unsystematic) names used by chemists (derived from names of ions)
Examples
HCl
HNO3
HNO2
H2SO4
H2SO3
hydrogen chloride
hydrogen nitrate
hydrogen nitrite
hydrogen sulfate
hydrogen sulfite
hydrochlorid acid (when dissolved as electrolyte)
nitric acid
(more oxygens use –ic)
nitrous acid (less oxygens use –ous)
sulfuric acid
sulfurous acid (less oxygens use –ous)
CaSO3 calcium sulfite
CaSO4 calcium sulfate (salt produced by neutralization Ca(OH)2+H2SO4→CaSO4+2H2O)
H2CO3
HF
Hydrogen carbonate
Hydro fluorid acid
carbonic acid
CH3
Scheme for naming ACIDS
No
Yes
Is there oxygen in
anion? Hn anion
Anion name ends
-ITE
-ATE
HYDRO anion – IC
Example
HF hydro fluoric acid
HBr hydro bromic acid
NO2 nitrite
SO3 sulfite
NO3 nitrate
SO4 sulfate
anion – OUS
anion – IC
Example
HNO2 nitrous acid
H2SO3 sulfurous acid
Example
HNO3 nitric acid
H2SO4 sulfuric acid
CH3
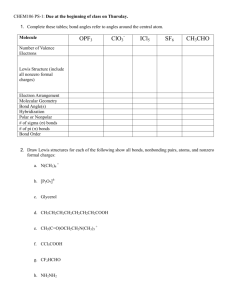
Lewis formula
Deeper insight into molecular structure is yielded by the Lewis electron-dot
formulas. Lewis symbols consist of the symbol for the element and one dot for
each electron in the valence shell (the symbol represents the kernel of
atom=nucleus+electrons not taking part in bonding). A dash is used to indicate
a shared pair of electrons instead of a colon (one dash denotes a single bond,
two dashes a double bond, and three dashes a triple bond).
water H2O
methane CH4
ethylene C2H4
benzene C6H6
H
C
HC
C H
H C
C
H
CH
CH3
Lewis formula
How to derive the Lewis formula, given a chemical formula.
1. Draw a possible structure(s) of molecule (e.g. O-C-O, or C-O-O for CO2)
2. Calculate total number of valence electrons
3. Try to distribute these electrons so that all elements fill their valence shell
(octet rule).
Water
methane CH4
ethylene C2H4
6+2=8 el.
4+4=8 el.
8+4=12 el.
benzene C6H6
24+6=30 el.
HC
H C
H
C
C
H
C H
CH
CH3
Lewis formula of ions
How to derive the Lewis formula, given a chemical formula and charge of ion.
1. Draw a possible structure(s) of ion
2. Calculate total number of valence electrons – charge of ion
3. Try to distribute these electrons so that all elements fill their valence shell
(octet rule).
Ammonium (NH4)+
Sulfate (SO4)2-
(5+4) - 1=8 electrons
(6+4x6) + 2=32 electrons
+
N
2-
O
O
S
O
O
.
CH3
Relax
CH3
MIXTURES - concentrations
CH3
MIXTURES - concentrations
CH3
MASS BALANCING
CH3
MASS BALANCING
Example: burner (continuous system) f-fuel, o-oxidiser, e-flue gas streams,
m f
Combustion
chamber, burners
mc
m o
Mass balance of mixture
m e
Mass flowrate [kg/s]. Streams are composed
of O2,N2,CO2,CO,CH4,H2O
m f m o m e
{inlet flowrate} = {outlet flowrate}
Mass balances of individual components (chemical compounds)
m O2 ,o = m O2 ,e m O
2
{inlet flowrate of O2 } = {outlet flowrate of O2 } + {rate of production of O 2 inside the black box}
O2 ,o m o O2 ,e m e m O2
Mass balances of elements (C,H,O,N - four equations)
m f
MC
MC
MC
m e (CH 4 , e
CO2 , e
)
M CH 4
M CH 4
M CO2
CH3
STOICHIOMETRY OF CHEMICAL REACTION
CH3
Tutorial - Stoichiometry
My car has consumption 9 l/100 km (fuel=octane). How many
kg of CO2 will be produced in my way Praha-Nachod?
Distance 290 km (use www.mapy.cz).
Octane CH3(CH2)6CH3 , density 703 kg/m3 (use www.wikipedia.com)
Answer: 56.7 kg CO2
Remark: gasoline is in fact a mixture of hydrocarbons, including aromatic
hydrocarbons like benzene (C6H6) and toluene (C6H5CH3). Octane is only one
of many component in the mixture.
solution: consumed Octane 2.9x9x0.703=18.35kg
mass fraction of C in octane 8x12/(8x12+6+2*6)=0.842
burned C 18.35x0.842=15.45 kg
Notice the fact that it was
not necessary to know the
chemical reaction (oxidation
of octane)!
12kgC corresponds to 44kgCO2. therefore 15.45/12x44=56.7 kg CO2
CH3
Tutorial - Stoichiometry
How many kg of air will be consumed on my way PrahaNachod (assuming ideal combustion of octane)?
Air: Mixture of O2 (21% volum.), N2 (79%)
Hint: It is necessary to calculate mass fraction of oxygen in air.
2C8H18 + 25O2 16CO2 + 18H2O
228kg
800kg
704kg
324kg
18.35kg
64.4kg
56.7kg
26.1 kg
There was consumed 64.4kg of pure oxygen.
CH3
Tutorial – naming and formula
SF6
Sulfur hexafluoride
Fe2O3
Iron (III) oxide
(NH4)2SO4
Ammonium sulfate (and not sulfite)
Al(NO3)3
Aluminium nitrate
HNO3
Nitric acid
HNO2
Nitrous acid
H2CO3
Carbonic acid
H2SO3
Sulfurous acid
H2SO4
Sulfuric acid
CuI
Copper(I) iodide
CaCO3
Calcium carbonate
CaSO4
Calcium sulfate
Na2SO3
Sodium sulfite
CH3
Tutorial Lewis formula
How to derive the Lewis formula, given a chemical formula and charge of ion.
1. Draw a possible structure(s) of ion
2. Calculate total number of valence electrons (– charge of ion)
3. Try to distribute these electrons so that all elements fill their valence shell
(octet rule).
CO2
CO
O=C=O
CO
N2