Electron Configuration Be
advertisement

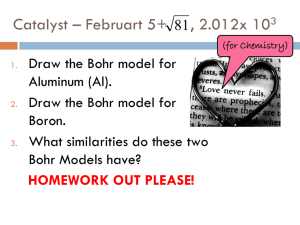
Catalyst – February 32, 2011 WAY BACK WEDNESDAY!!! 1. 2. 3. Which layer is oldest: 23, 24, 25, or 31? Which layer is youngest: 23, 24, 25, or 31? Which occurred last: Lava Flow A, Lava Flow B, or Sill/Magma Chamber? Today’s Agenda Catalyst Review Electron Configuration Overview of Project Exit Question HW: STUDY FOR TEST, RESEARCH ELEMENT Today’s Objectives SWBAT write long electron configurations and noble gas configurations for elements. SWBAT begin their element project! How? Schrödinger Equation REVIEW: The Schrödinger Equation predicts the location of an electron in an atom. It tells us that electrons hang out in atomic orbitals. Imagine electrons filing into hotels… Schrödinger Equation and Atomic Orbitals REVIEW: There are 4 atomic orbitals… s p d f The s Orbital One s orbital in each energy level The s orbital is shaped like a sphere Can hold 2 electrons total The p Orbital Three p orbitals in each energy level (starting in the 2nd energy level!) Shape looks like dumbells or bowties Can hold 6 electrons total The d orbital -Five d orbitals in each energy level (starting in the 3rd energy level!) -Shape looks like eggs or flowers -Can hold 10 electrons The f orbital Seven f orbitals (starting in 4th energy level!) Can hold 14 electrons So how many electron fit in the THIRD energy level??? 32 18 8 2 Electron Configuration REVIEW: Electron configuration shows how the electrons are distributed among the various atomic orbitals and energy levels. The format consists of a series of numbers, letters, and superscripts as shown below: 1s2 The Code Small superscript number = Number of electrons 2 1s Large number = energy level Letter = orbital Na – Electron Configuration 2 2 6 1 1s 2s 2p 3s = 11 e- Electron Configuration Be: Ar: Zn: Electron Configuration Be: 2 2 1s 2s Ar: 2 2 6 2 6 1s 2s 2p 3s 3p Zn: 1s22s22p63s23p64s23d10 Noble Gas Configuration Scientists are WAY too lazy to write all that mess! Electron Configuration FIGURE IT OUT YOURSELF!!! (10 mins) 2 14 10 [Xe]6s 4f 5d Hg – 2 4 S – [Ne]3s 3p 2 5 Sm – [Xe]6s 4f SiRb- Noble Gas Configuration Key Point #4: Noble Gas configuration starts with noble gas BEFORE the atom you are going to. Examples of Noble Gas Configuration Long Electron Configuration Noble Gas Configuration 2 1 Al – 1s22s22p63s23p1 Al – [Ne]3s 3p 2 2 Ti – 1s22s22p63s23p64s23d2 Ti – [Ar]4s 3d Rb – 1s22s22p63s23p64s23d104p65s1 Rb – [Kr]5s1 Now you try… Project Overview Exit Question 1. 2. What is your element for the project? What topics do you need to review the most before the test on Friday?