The drug or other substance has a currently accepted medical use in
advertisement

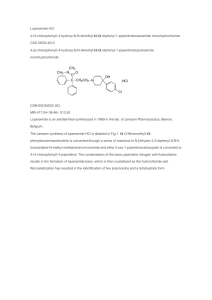
Summary of structure-activity relationships (SAR’s) Removal of OH reduces activity 2 HO 3 11 4 Removal increases activity 10 15 12 O 16 9 13 5 14 H H HO N-CH2CH2Ph increases N-CH2CH=CH2 creates antagonist 1 8 6 N CH3 7 Introduction of OH increases activity Oxidation, coupled with reduction of 7,8 C=C, increases activity Acetylation increases activity Reduction increases activity What structural elements are necessary for activity? Aromatic Ring Spacer 2 HO 3 1 11 4 12 O 10 15 9 13 5 14 H H HO 16 8 6 N R1 CH3 R2 N R3 CH3 7 Quaternary Carbon Center Basic Nitrogen Removing the oxide bridge (and hydrogenating double bond, removing one alcohol) produces levorphanol, which has enhanced analgesic properties over morphine. HO 3 1 10 15 12 11 4 11 13 H 8 6 7 16 9 14 H 10 15 12 16 9 5 HO 3 1 4 O 2 HO 2 N 13 5 CH3 14 H N H 8 6 CH3 7 Levorphanol Levorphanol is used to treat severe pain and has several brand names. Generic Name Levorphanol Brand Names/Synonyms Antalgin Aromarone Cetarin Dea No. 9220 Dea No. 9733 Dromoran Lemoran Levo-Dromoran Levorfanol [Inn-Spanish] Levorfanolo [Dcit] Levorphan Levorphanal Levorphanol Dl-Form Levorphanol Tartrate Levorphanolum [Inn-Latin] Methorfinan [Czech] Methorphinan Orphan Racemethorphanum Racemic Dromoran Racemorfano [Inn-Spanish] Racemorphan Racemorphan [Ban:Inn] Racemorphane [Inn-French] Racemorphanum [Inn-Latin]anum [Inn-Latin] Surprisingly, its mirror image still has antitussive properties, but no analgesic properties 2 1 11 16 10 3 1 11 4 4 15 2 HO OH 3 12 12 H3C 16 9 9 N 10 15 14 H 13 13 5 5 14 H N H H 8 8 6 6 CH3 7 7 Levorphanol dextrorphan analgesic + antitussive Antitussive only Mirror Methylating the phenolic hydroxyl group improves this antitussive activity 2 11 16 10 OCH3 3 1 4 15 12 9 N H 3C 14 H 13 5 H 8 6 7 Dextromethorphan (DM) Antitussive only Dextromethorphan (DM or DXM) is an antitussive drug that is found in many over-the-counter cold and cough preparations, usually in the form of dextromethorphan hydrobromide. It is also commonly taken above the recommended dosage by users seeking its dissociative effect. • • • The FDA has approved dextromethorphan for over-the-counter sale as a cough suppressant. A combination of dextromethorphan and quinidine has been shown to alleviate symptoms of easy laughing and crying (pseudobulbar affect) in patients with amyotrophic lateral sclerosis and multiple sclerosis.[3] Dextromethorphan is being investigated as a possible treatment for pain associated with fibromyalgia, a chronic rheumatological organic fatigue disorder.[4] Dextromethorphan is also useful in breaking addictions to narcotics and other habit-forming drugs (including nicotine), since it is an inhibitor of many of the brain receptors involved in narcotic action on the brain. For this purpose, DXM is more effective when combined with an oxidase inhibitor, which suppresses its inactivation and increases its half-life; thus it increases the concentration of DXM in the circulating blood and extends its effective duration.[5] Aromatic Ring Spacer 2 HO 3 1 11 4 12 O 10 15 9 13 5 14 H H HO 16 8 6 N R1 CH3 R2 N R3 CH3 7 Quaternary Carbon Center O N O CH3 Et Meperidine (DemerolTM) (PethidineTM) Basic Nitrogen Meperidine • Pethidine (INN) or meperidine (USAN) (also referred to as: isonipecaine; lidol; pethanol; piridosal; Algilィ; Alodanィ; Centralginィ; Demerolィ; Dispadolィ; Dolantinィ; Dolarganィ (in Poland);[1] Dolestineィ; Dolosalィ; Dolsinィ; Mefedinaィ) is a fast-acting opioid analgesic drug. In the United States, it is more commonly known as meperidine or by its brand name Demerol.[2]Pethidine is indicated for the treatment of moderate to severe pain, and is delivered as its hydrochloride salt in tablets, as a syrup, or by intramuscular or intravenous injection. Meperidine • For much of the 20th century, pethidine was the opioid of choice for many physicians; in 1983 60% of doctors prescribed it for acute pain and 22% for chronic severe pain.[3] Compared to morphine, pethidine was supposed to be safer and carry less risk of addiction, and to be superior in treating the pain associated with biliary spasm or renal colic due to its putative antispasmodic effects. In fact, pethidine is no more effective than morphine at treating biliary or renal pain, and its low potency, short duration of action, and unique toxicity (i.e. seizures, delirium, other neuropsychological effects) relative to other available opioid analgesics have seen it fall out of favor in recent years, for all but a very few, very specific indications. Several countries, including Australia, have put severe limits on its use or curtailed it outright.[4] Nevertheless, some physicians continue to use it as a first-line strong opioid. Opioids to treat diarrhea? Aromatic Ring Spacer 2 HO 3 1 11 4 12 O 10 15 16 9 13 5 14 N H R1 CH3 H HO 8 6 R2 N R3 CH3 7 Quaternary Carbon Center Basic Nitrogen Cl O N HO O O Et N O N CN N O CH3 CH3 CH3 Et Meperidine (DemerolTM) (PethidineTM) Diphenoxylate (active ingredient of Lomotil) (mixture with atropine, to prevent abuse) Loperamide (active ingredient of Imodium) Does not cross BBB, thus no analgesic effect Diphenoxylate • Diphenoxylate is an opioid agonist used for the treatment of diarrhea that acts by slowing intestinal contractions. It was discovered at Janssen Pharmaceutica in 1956. It is a congener to the narcotic Meperidine of which the common brand name is Demerol. This being the case, this medication is potentially habit-forming, particularly in high doses or when long-time usage is involved. Because of this, diphenoxylate is manufactured and marketed as a combination drug with atropine (Lomotilィ). Lomotil • This pharmaceutical strategy is designed to discourage abuse, because the anticholinergic effect of atropine will produce severe weakness and nausea if standard dosage is exceeded. • Tablets - round, white, with SEARLE debossed on one side and 61 on the other side and containing 2.5 mg of diphenoxylate hydrochloride and 0.025 mg of atropine sulfate, Loperamide (Imodium) • Loperamide is an opioid receptor agonist and acts on the μ-opioid receptors in the myenteric plexus large intestines; it does not affect the central nervous system like other opioids. Loperamide (Imodium) • It works by decreasing the activity of the myenteric plexus which decreases the motility of the circular and longitudinal smooth muscles of the intestinal wall. This increases the amount of time substances stay in the intestine, allowing for more water to be absorbed out of the fecal matter. Loperamide also decreases colonic mass movements and suppresses the gastrocolic reflex.[1] Loperamide (Imodium) • Loperamide does not cross the bloodbrain barrier and has no analgesic properties. Tolerance in response to longterm use has not been reported.However, loperamide can cause physical dependence. Symptoms of opiate withdrawal have been observed in patients abruptly discontinuing long-term therapy with loperamide. Fentanyl Aromatic Ring Spacer 2 HO 3 1 11 4 O 10 15 12 9 14 13 5 H H HO 16 8 6 N R1 R2 CH3 N R3 CH3 7 Quaternary Carbon Center O O N O N CH3 Basic Nitrogen N CH2CH2Ph Et Et Meperidine (DemerolTM) (PethidineTM) Fentanyl (80X more potent than morphine!) Fentanyl N O N CH2CH2Ph Et • Fentanyl is an opioid analgesic, first synthesized by Janssen Pharmaceutica (Belgium) in the late 1950s, with an analgesic potency of about 80 times that of morphine. Fentanyl was introduced into medical practice in the 1960s as an intravenous anesthetic under the trade name of Sublimaze. Fentanyl analogs O OCH3 N O O N O N CH2CH2Ph N Et N Alfentanil (Alfenta) Fentanyl (80X more potent than morphine!) N Et Remifentanil N OCH3 O O N S Et Sufentanil (Sufenta) 5X more potent than Fentanil N OCH3 N Et N O N OCH3 N O OCH3 N Et Carfentanil (100X more potent than Fentanil!) O Fentanyl and analogs • Fentanyls are extensively used for anesthesia and analgesia, most often in the operating room and intensive care unit. Duragesic, by Janssen Pharmaceutica, is a fentanyl transdermal patch used in chronic pain management. Duragesic patches work by releasing fentanyl into body fats, which then slowly release the drug into the blood stream over 72 hours, allowing for long lasting relief from pain. Carfentanil • Carfentanil was discovered by Janssen Pharmaceutica. It has a quantitative potency approximately 10,000 times that of morphine and 100 times that of fentanyl, activity in humans starting at about 1 μg. It is marketed under the trade name Wildnil as a tranquilizer for large animals.[1] Carfentanil is intended for animal use only as its extreme potency makes it inappropriate for use in humans. Carfentanil • It is thought that in the 2002 Moscow theater hostage crisis, the Russian military made use of an aerosol form of carfentanil to subdue Chechen hostage takers.[2] Its short action, easy reversibility and therapeutic index (10600 vs. 300 for fentanyl) would make it a near-perfect agent for this purpose. Carfentanil • Wax et al. surmise from the available evidence that the Moscow emergency services had not been informed of the use of the agent, and therefore did not have adequate supplies of naloxone or naltrexone (opioid antagonists) to prevent complications in many of the victims. Assuming that carfentanil was the only active constituent (which has not been verified by the Russian military), the primary acute toxic effect to the theatre victims would have been opioid-induced apnea; in this case mechanical ventilation and/or treatment with opioid antagonists would have been life-saving for many or all victims. Methadone Aromatic Ring Spacer 2 HO 3 1 11 4 12 O 10 15 9 13 5 14 H H HO 16 8 6 N R1 CH3 R2 N R3 CH3 7 Quaternary Carbon Center CH3 O CH3 N CH3 Methadone Basic Nitrogen Methadone • Methadone/dolophine, was first synthesized in 1937 by German scientists Max Bockm殄l and Gustav Ehrhart at IG Farben (Hoechst-Am-Main, now part of Frankfurt, Germany) during their search for an analgesic that would be easier to use during surgery (and less potentially addictive, post-op) than morphine. Methadone CH3 O CH3 N CH3 • Methadone was introduced into the United States in 1947 by Eli Lilly and Company as an analgesic (They gave it the trade name Dolophineィ, which is now registered to Roxane Laboratories). Since then, it has been best known for its use in treating narcotic addiction, although such a use never became widespread and common until the early 1990's when public policy sought to find ways to reduce the spread of HIV and AIDS. ‘Scheduling’ of Drugs • Drugs are classified or “scheduled” depending on their potential for abuse and whether or not there is a medical need for a particular drug. Schedule I drugs • Findings required: • (A) The drug or other substance has a high potential for abuse. • (B) The drug or other substance has no currently accepted medical use in treatment in the United States. • (C) There is a lack of accepted safety for use of the drug or other substance under medical supervision. • Examples include Heroin, Cannabis, and LSD Schedule II drugs Findings required: (A) The drug or other substance has a high potential for abuse. (B) The drug or other substance has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. (C) Abuse of the drug or other substances may lead to severe psychological or physical dependence. Examples include Morphine, Cocaine, Methylphenidate (Ritalin) Schedule III drugs • Findings required: • (A) The drug or other substance has a potential for abuse less than the drugs or other substances in schedules I and II. • (B) The drug or other substance has a currently accepted medical use in treatment in the United States. • (C) Abuse of the drug or other substance may lead to moderate or low physical dependence or high psychological dependence. • Examples include anabolic steroids, hydrocodone, and codeine. Schedule IV drugs • Findings required: • (A) The drug or other substance has a low potential for abuse relative to the drugs or other substances in schedule III. • (B) The drug or other substance has a currently accepted medical use in treatment in the United States. • (C) Abuse of the drug or other substance may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in schedule III. • Examples include: benzodiazepines and barbituates. Schedule V drugs • Findings required: • (A) The drug or other substance has a low potential for abuse relative to the drugs or other substances in schedule IV. • (B) The drug or other substance has a currently accepted medical use in treatment in the United States. • (C) Abuse of the drug or other substance may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in schedule IV. • Examples include cough syrups containing a small amount of codeine, or anti-diarrheal’s containing small amounts of diphenoxylate. Introduction to influenza • http://microbiology.mtsinai.on.ca/sarswatch/ presentations/index.asp • http://www.pharmasquare.org/flash/Tami flu.html